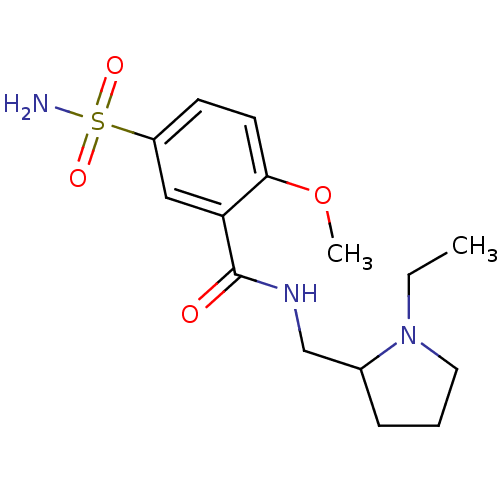
Affinity DataKi: 31nM ΔG°: -42.4kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic...More data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Inhibition of human membrane bound carbonic anhydrase 9 preincubated for 15 mins at room temperature followed by 72 hrs at 4 degC by stopped flow CO2...More data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Inhibition of human transmembrane carbonic anhydrase 9 by stopped-flow CO2 hydration assayMore data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Inhibition of catalytic domain of human recombinant CA IXMore data for this Ligand-Target Pair