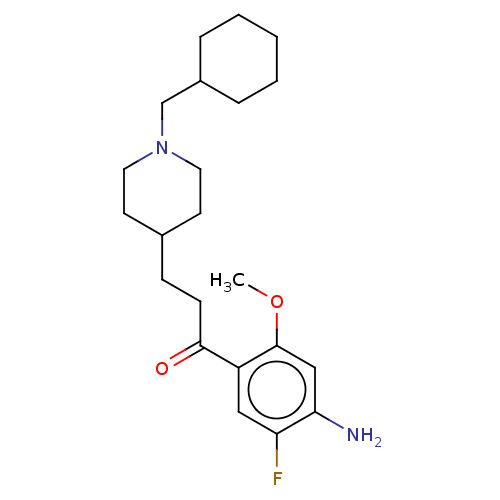
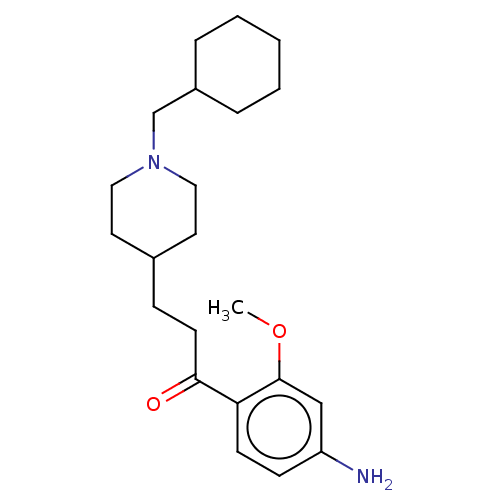
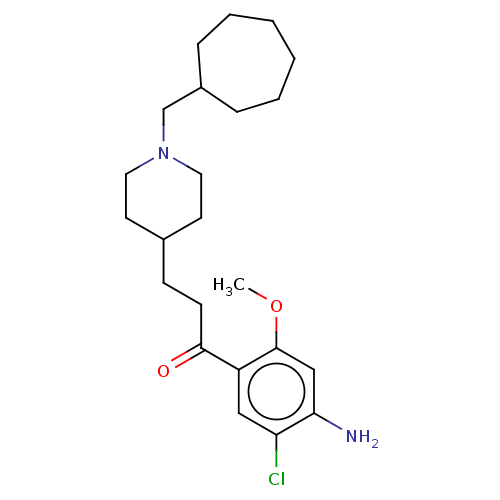
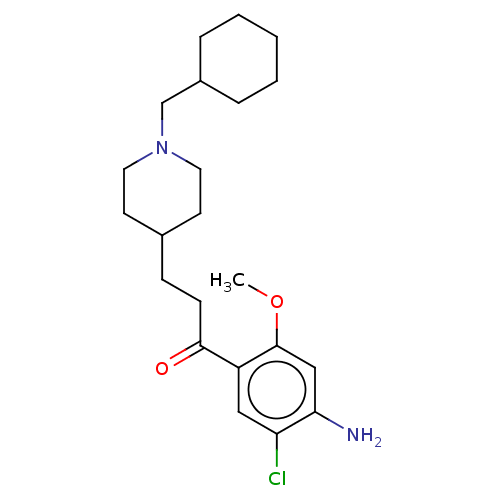
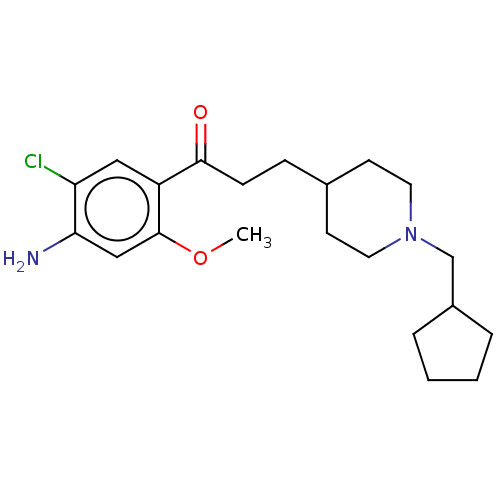
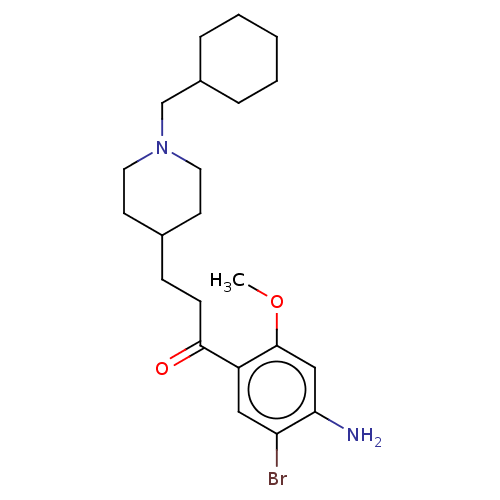
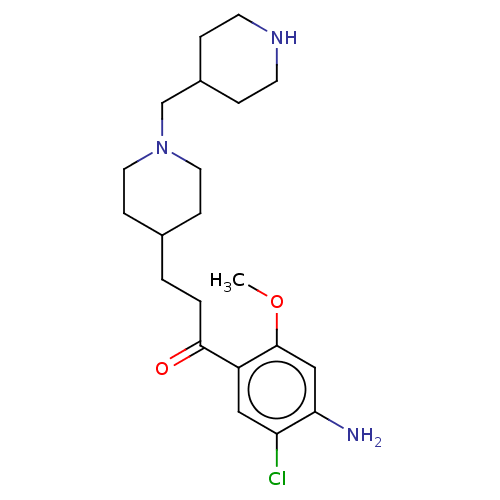
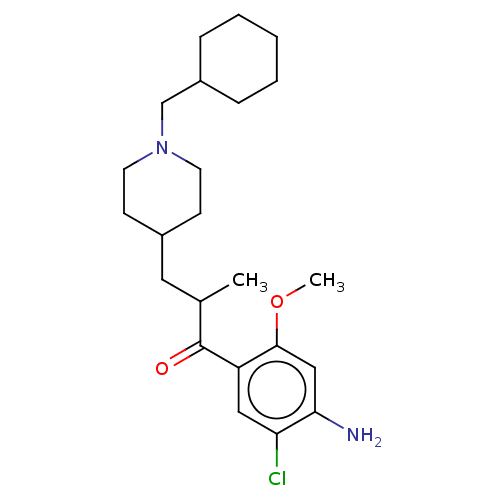
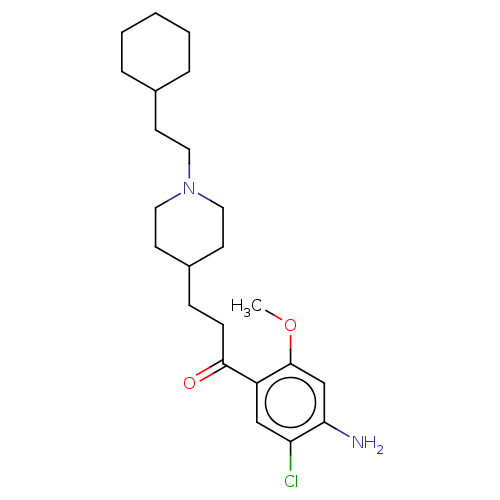
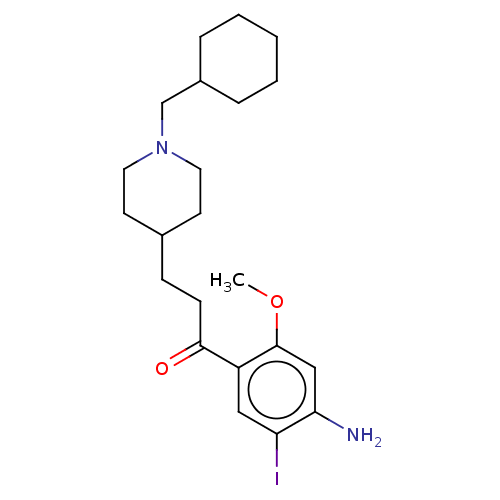
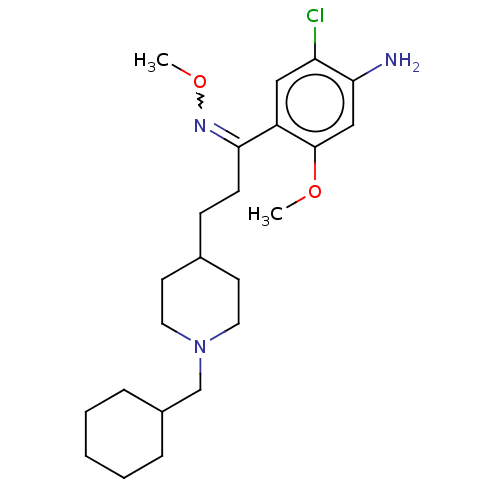
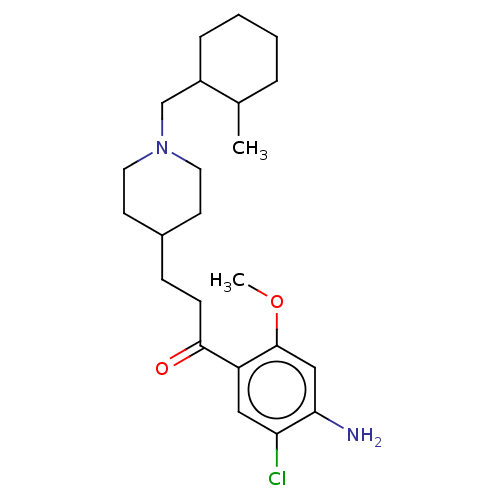
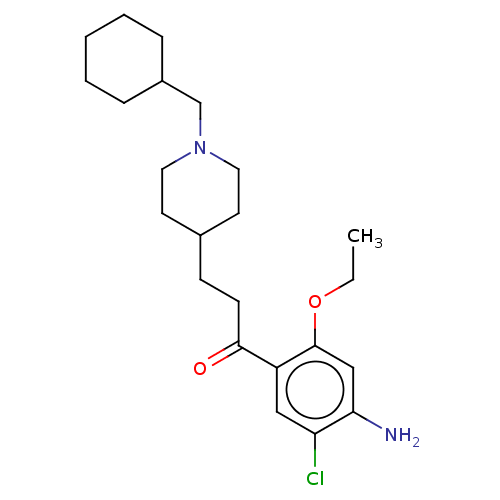
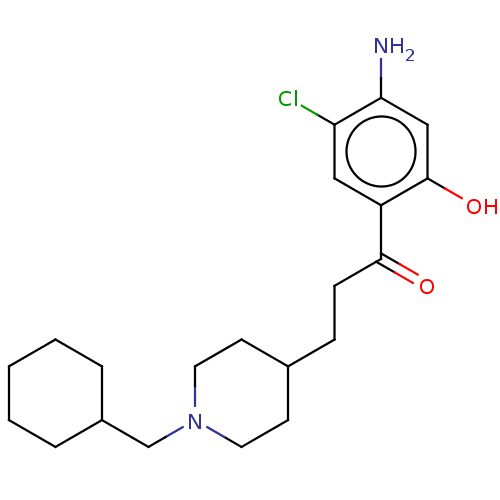
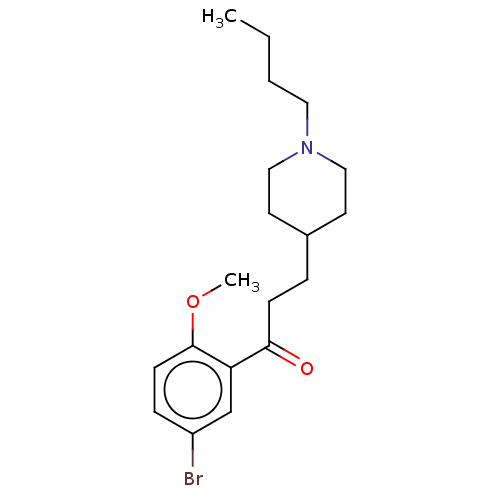
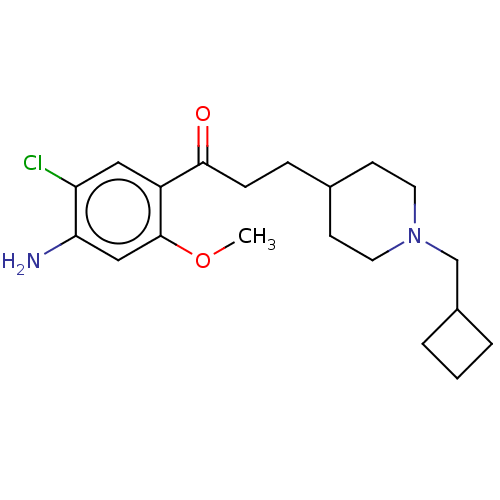
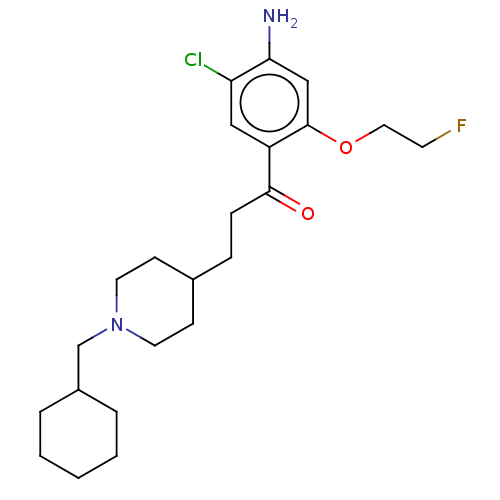
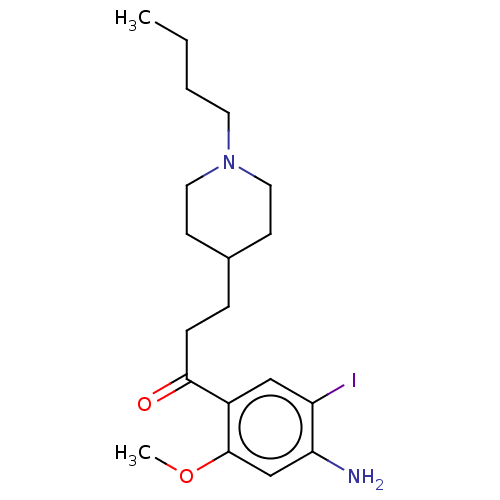
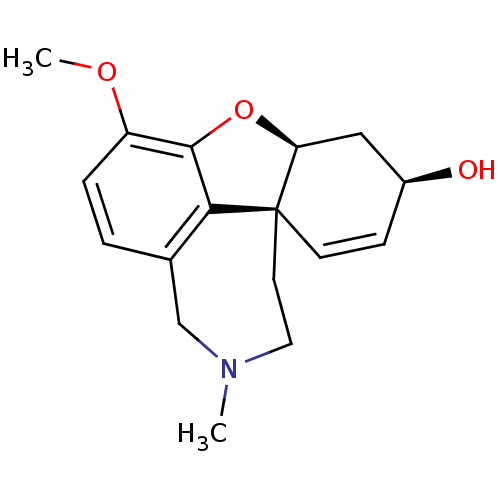
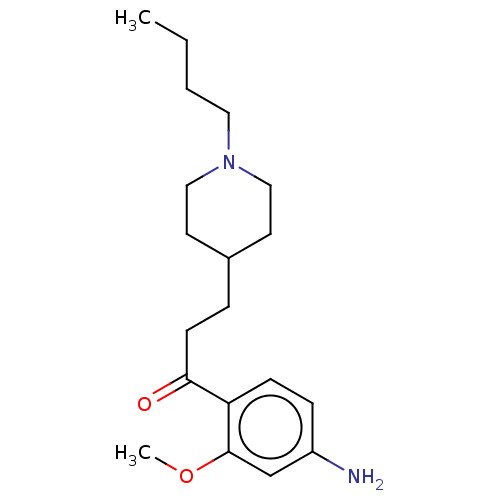
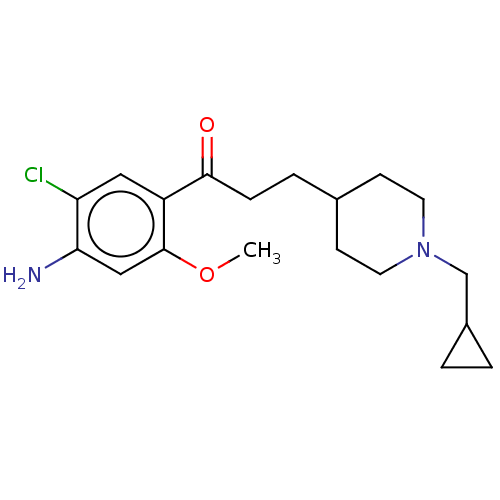
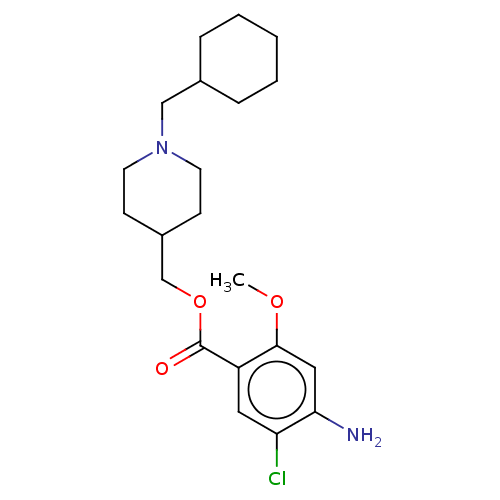
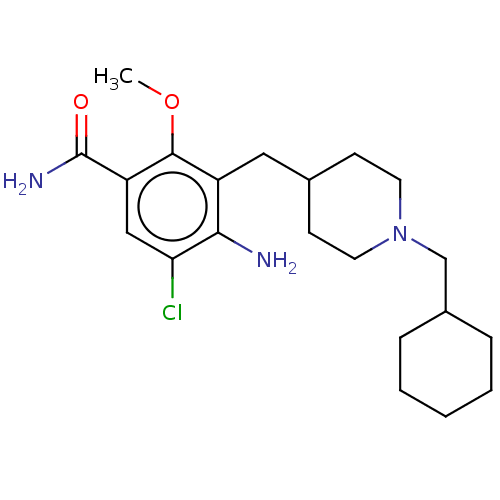
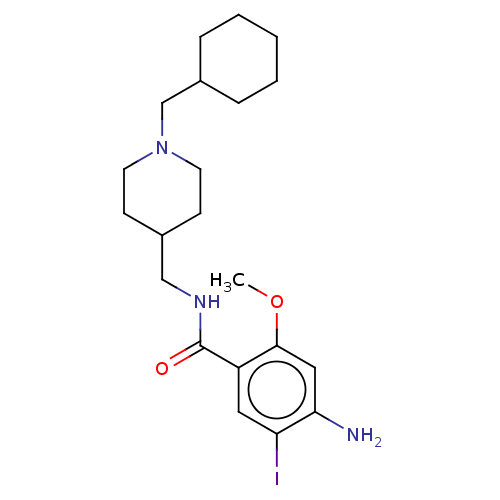
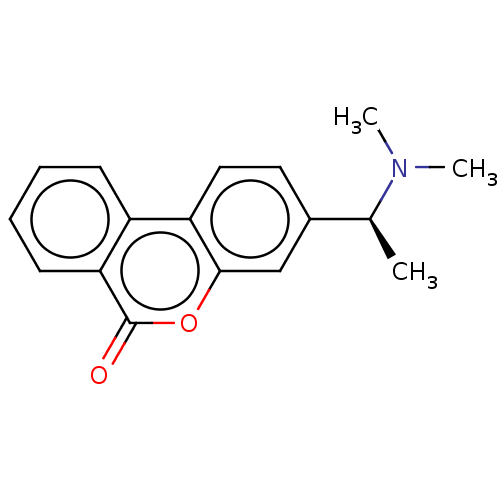
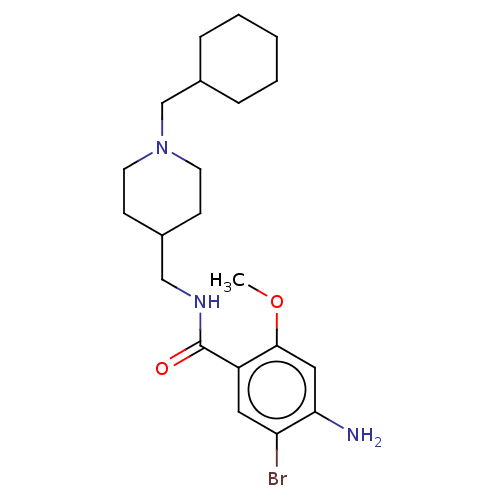
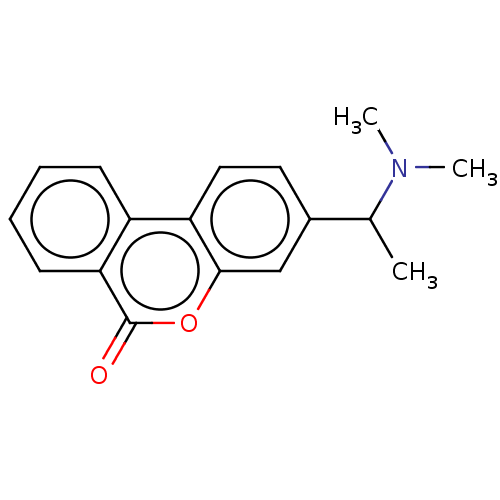
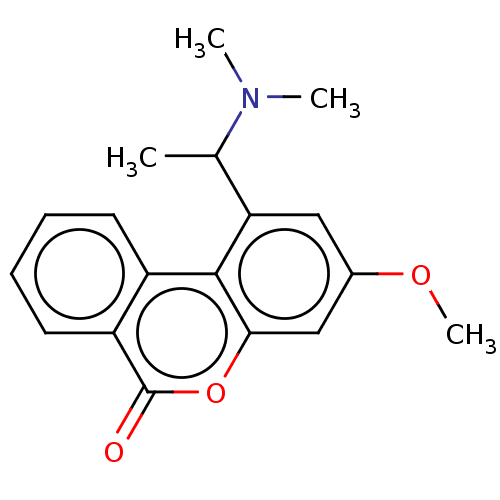
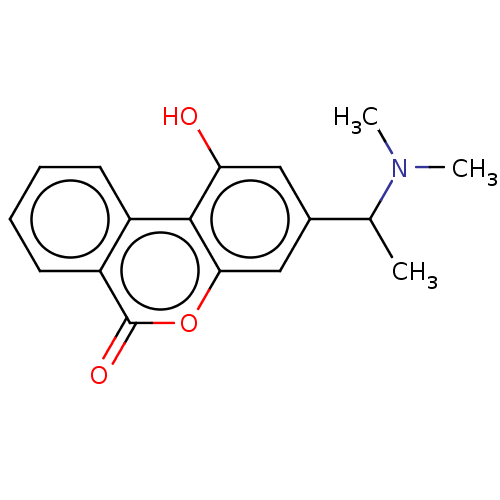
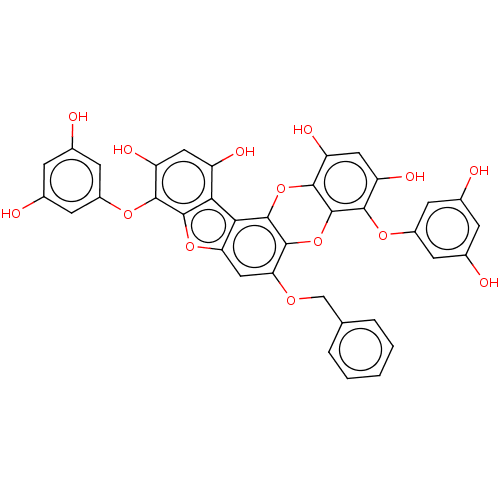
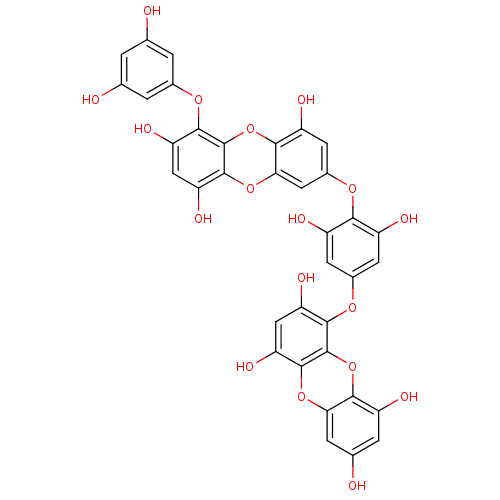
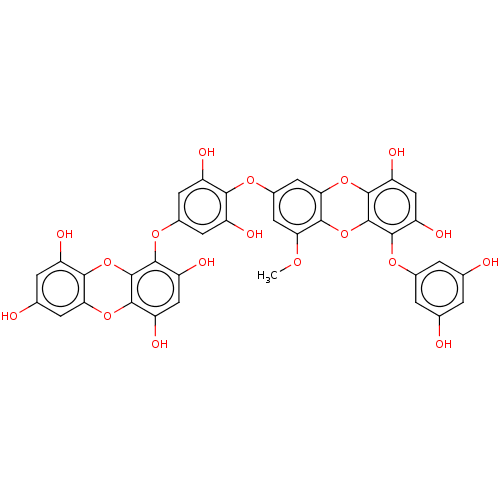
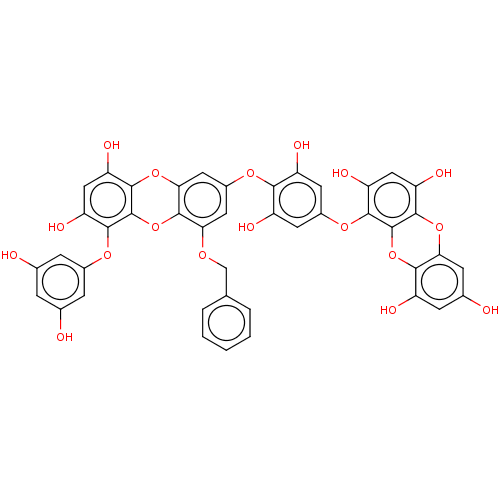
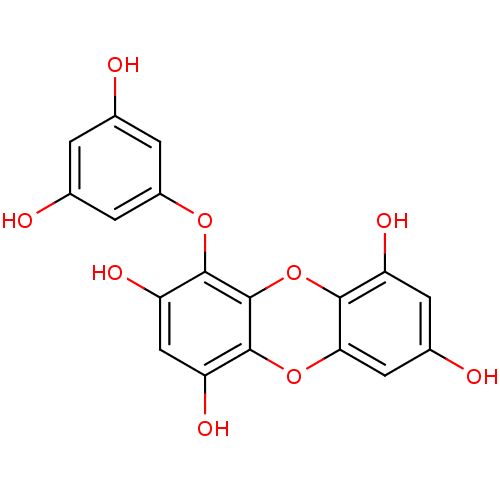
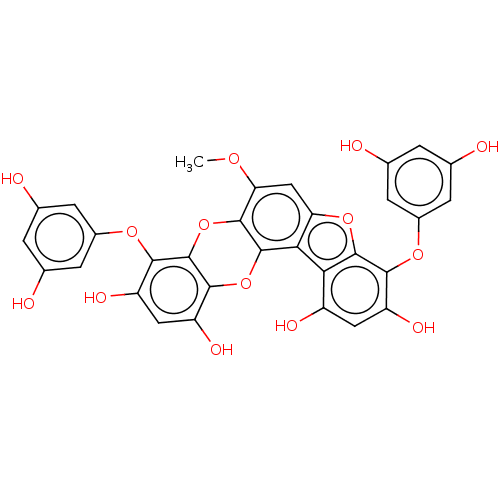
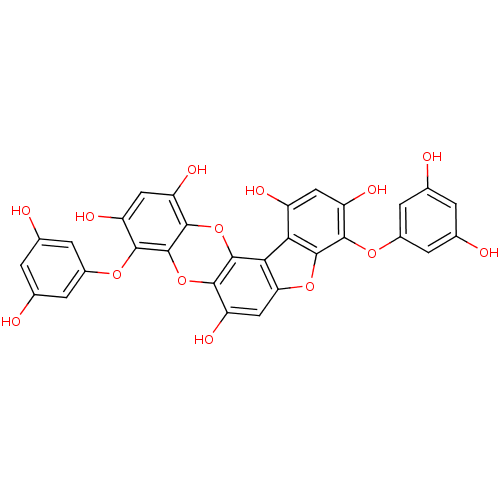
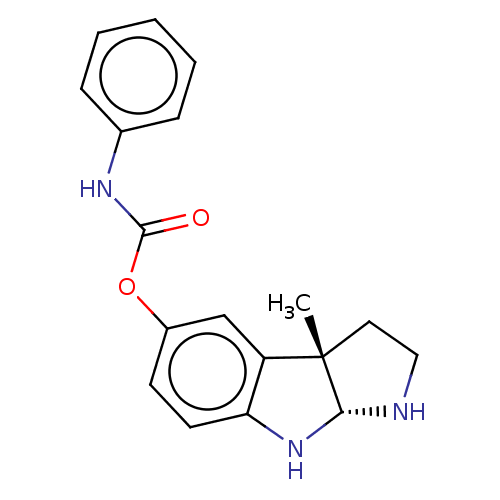
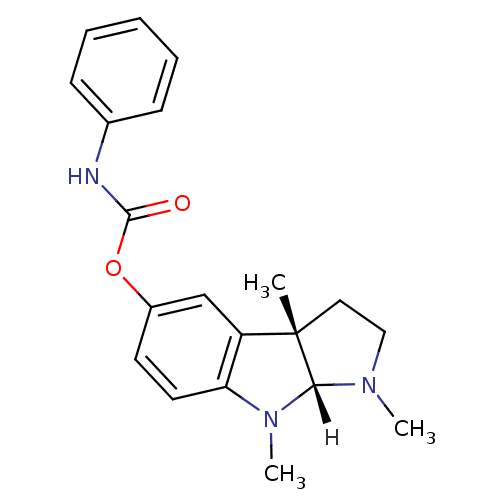
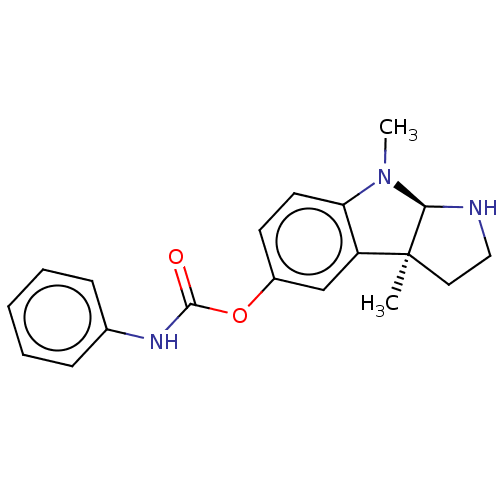
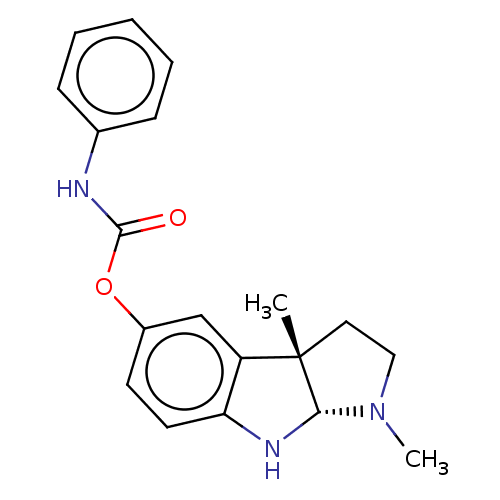
TargetAcetylcholinesterase(Rattus norvegicus (rat))
Pudue Pharma Discovery Research
Curated by PDSP Ki Database
Pudue Pharma Discovery Research
Curated by PDSP Ki Database
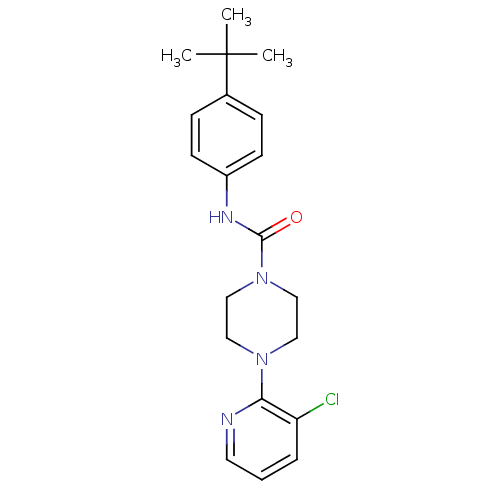
Affinity DataIC50: 8nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
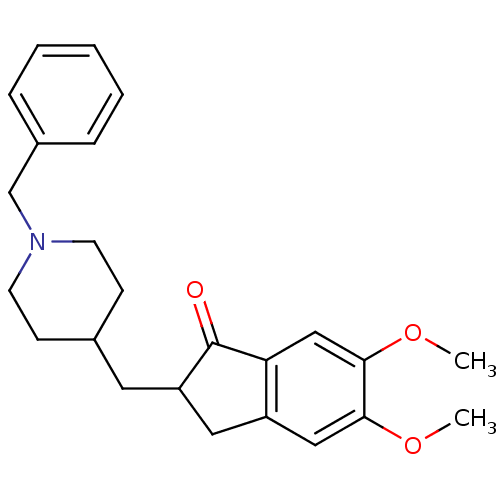
Affinity DataIC50: 11nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 63nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 69nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 99nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 118nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 201nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 222nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 304nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 320nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 321nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 395nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 411nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 445nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 577nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 625nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 658nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 748nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 937nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 2.72E+3nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 3.73E+3nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 5.09E+3nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 7.90E+3nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 8.70E+3nMAssay Description:Acetylcholinesterase extracted from human erythrocytes (buffered aqueous solution, ≧500 units/mg, Sigma Aldrich) is diluted in a 20 mM HEPES bu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.09E+4nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+4nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 1.89E+4nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 2.32E+4nMpH: 7.4 T: 2°CAssay Description:Enzyme activities were determined at room temperature. Ultraviolet absorbance was measured spectrophotometrically by a modification of a previously d...More data for this Ligand-Target Pair
Affinity DataIC50: 2.34E+4nMpH: 7.4 T: 2°CAssay Description:Enzyme activities were determined at room temperature. Ultraviolet absorbance was measured spectrophotometrically by a modification of a previously d...More data for this Ligand-Target Pair
Affinity DataIC50: 3.31E+4nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 3.32E+4nMpH: 7.4 T: 2°CAssay Description:Enzyme activities were determined at room temperature. Ultraviolet absorbance was measured spectrophotometrically by a modification of a previously d...More data for this Ligand-Target Pair
Affinity DataIC50: 3.42E+4nMpH: 7.4 T: 2°CAssay Description:Enzyme activities were determined at room temperature. Ultraviolet absorbance was measured spectrophotometrically by a modification of a previously d...More data for this Ligand-Target Pair
Affinity DataIC50: 3.52E+4nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+4nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:The potential of the compounds of the present invention to inhibit acetylcholinesterase and butyrylcholinesterase enzymes were tested according to th...More data for this Ligand-Target Pair
Affinity DataIC50: 4.01E+4nMpH: 7.4 T: 2°CAssay Description:Enzyme activities were determined at room temperature. Ultraviolet absorbance was measured spectrophotometrically by a modification of a previously d...More data for this Ligand-Target Pair
Affinity DataIC50: 4.19E+4nMpH: 7.4 T: 2°CAssay Description:Enzyme activities were determined at room temperature. Ultraviolet absorbance was measured spectrophotometrically by a modification of a previously d...More data for this Ligand-Target Pair
Affinity DataIC50: 4.51E+4nMpH: 7.4 T: 2°CAssay Description:Enzyme activities were determined at room temperature. Ultraviolet absorbance was measured spectrophotometrically by a modification of a previously d...More data for this Ligand-Target Pair
Affinity DataIC50: 6.56E+4nMpH: 7.4 T: 2°CAssay Description:Enzyme activities were determined at room temperature. Ultraviolet absorbance was measured spectrophotometrically by a modification of a previously d...More data for this Ligand-Target Pair
Affinity DataIC50: 9.41E+4nMpH: 7.4 T: 2°CAssay Description:Enzyme activities were determined at room temperature. Ultraviolet absorbance was measured spectrophotometrically by a modification of a previously d...More data for this Ligand-Target Pair
Affinity DataEC50: 100nMAssay Description:To address these issues, the present disclosure relates to the development and utilization of a (−)-phenserine extended release formulation. To...More data for this Ligand-Target Pair
Affinity DataEC50: 25nMAssay Description:To address these issues, the present disclosure relates to the development and utilization of a (−)-phenserine extended release formulation. To...More data for this Ligand-Target Pair
Affinity DataEC50: 62nMAssay Description:To address these issues, the present disclosure relates to the development and utilization of a (−)-phenserine extended release formulation. To...More data for this Ligand-Target Pair
Affinity DataEC50: 62nMAssay Description:To address these issues, the present disclosure relates to the development and utilization of a (−)-phenserine extended release formulation. To...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)