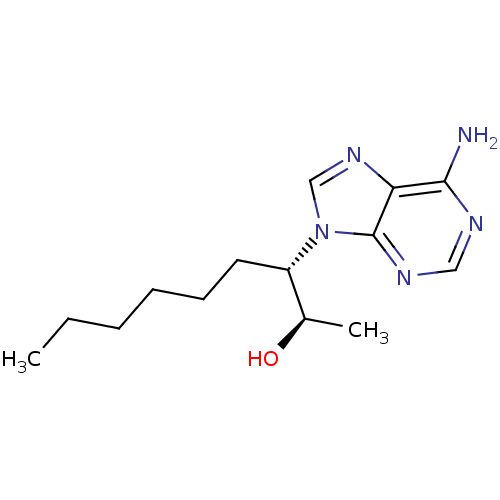
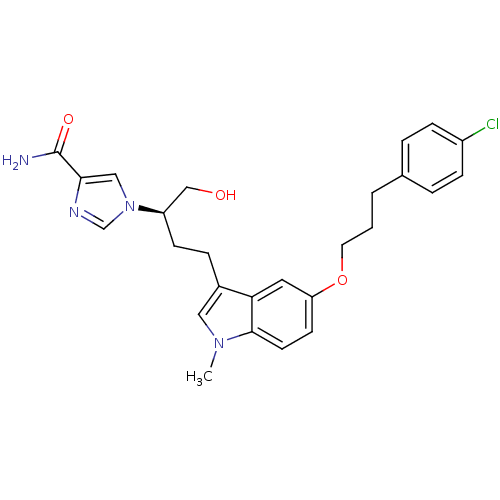
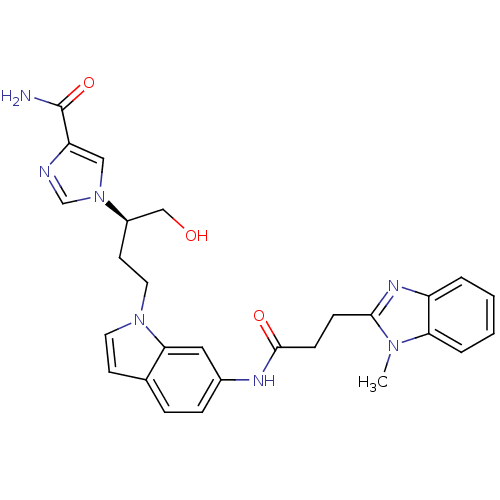
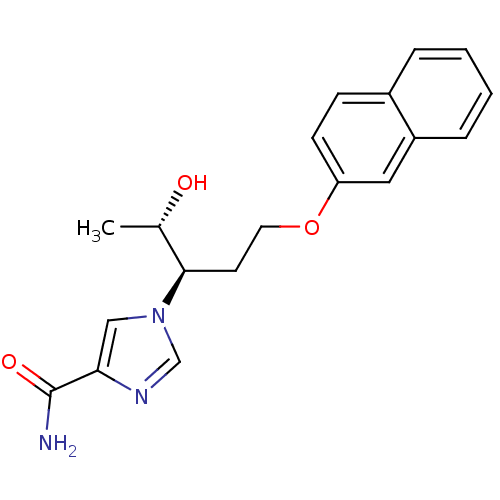
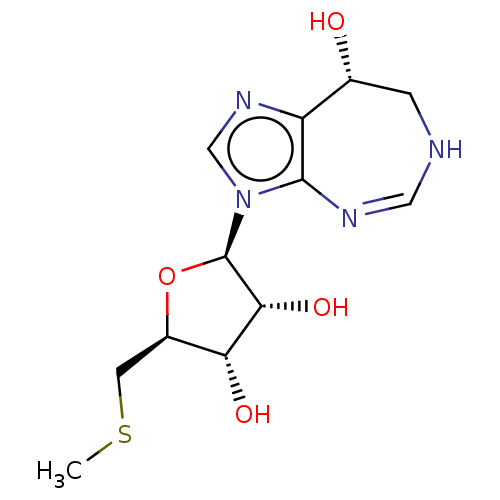
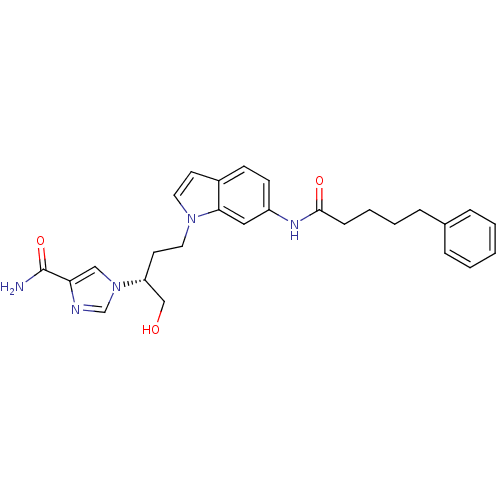
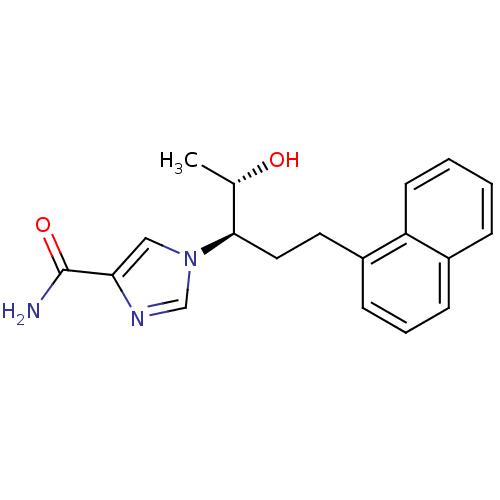
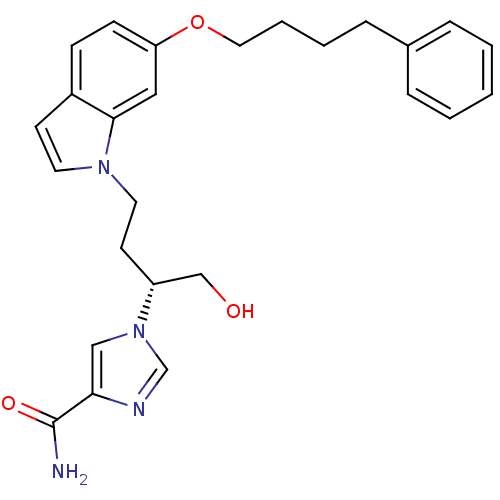
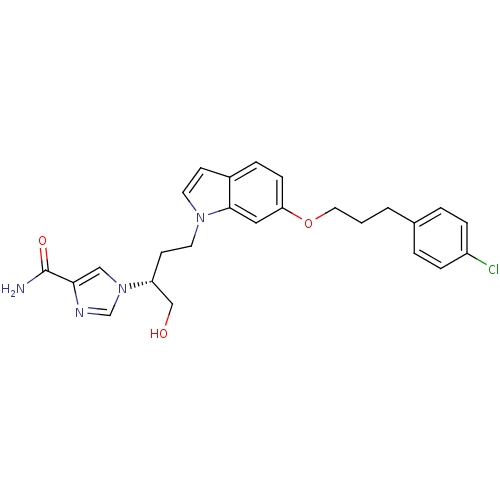
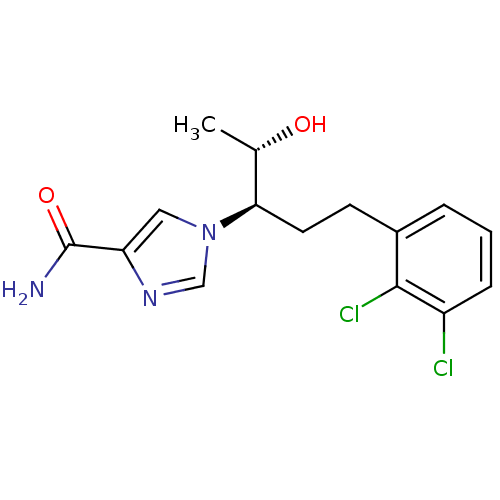
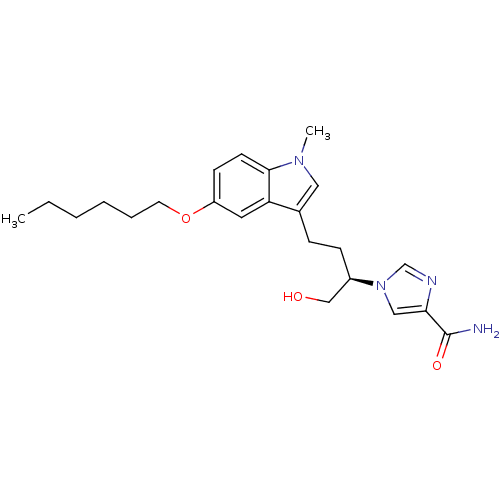
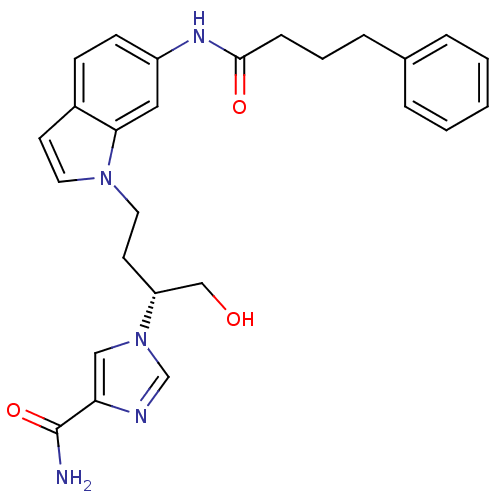
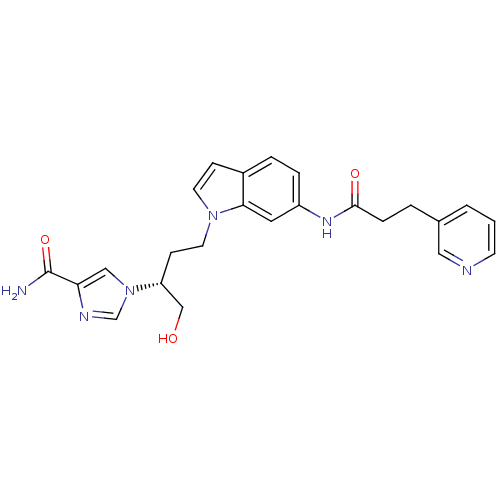
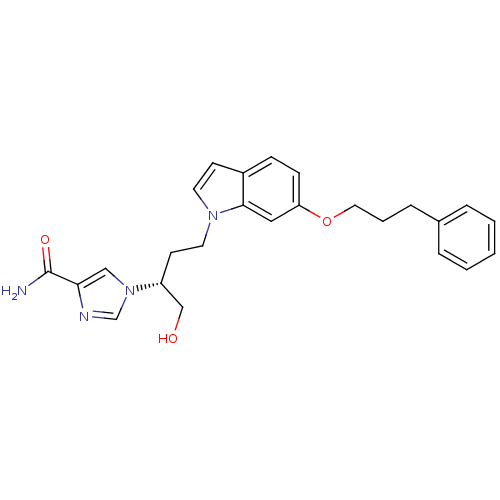
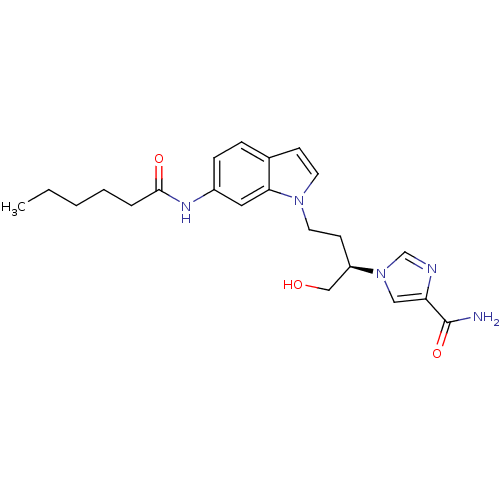
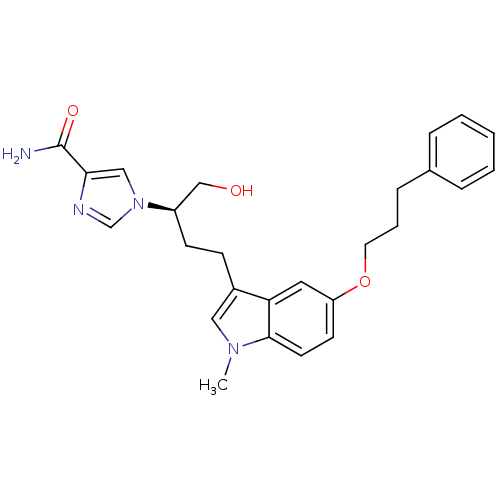
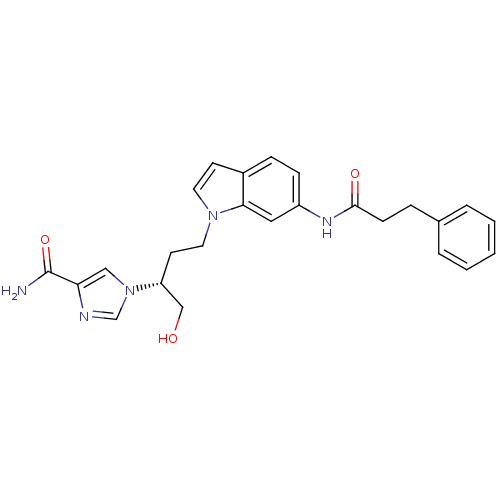
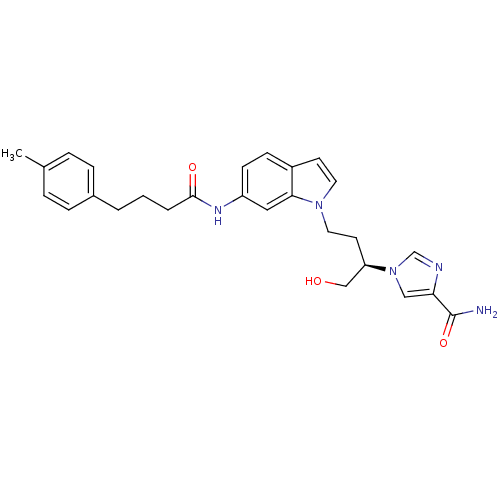
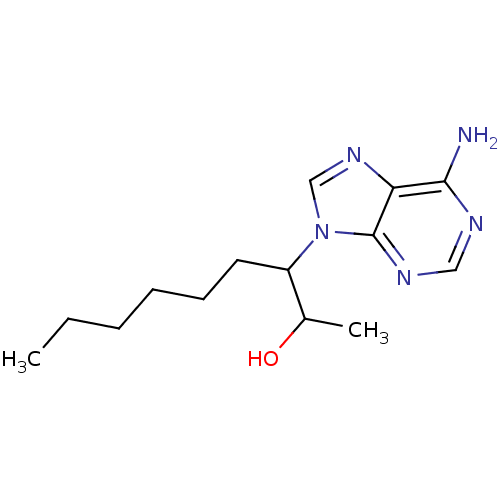
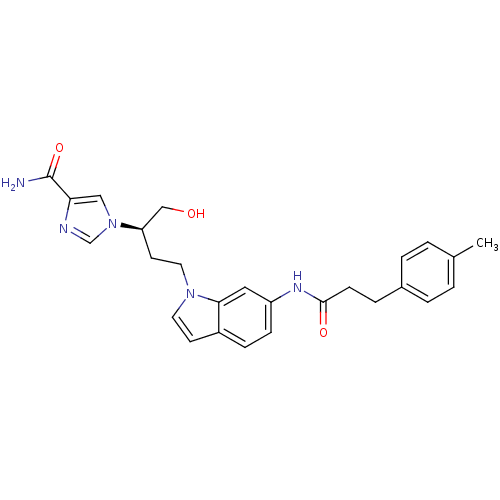
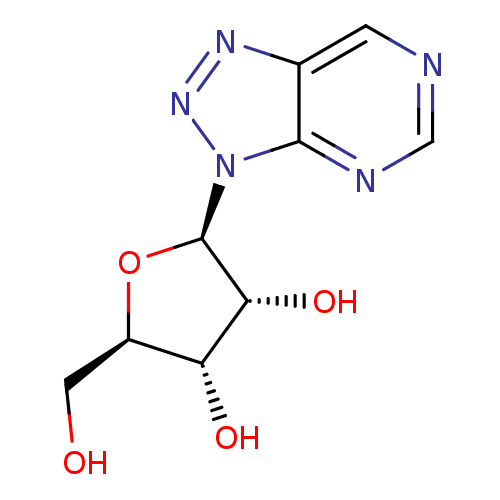
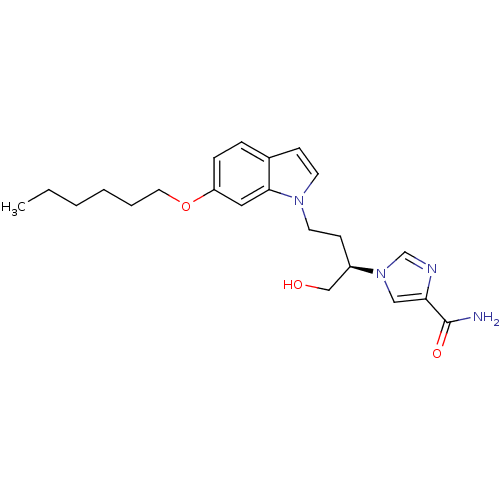
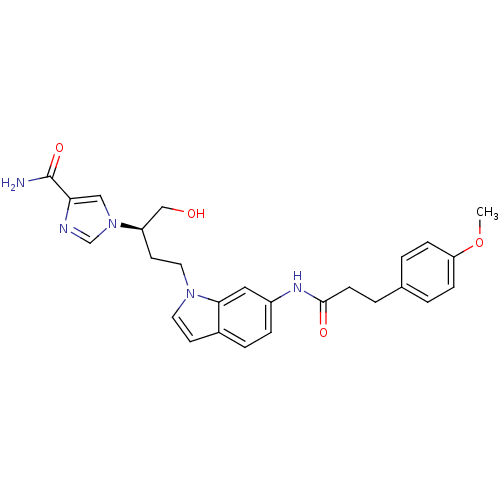
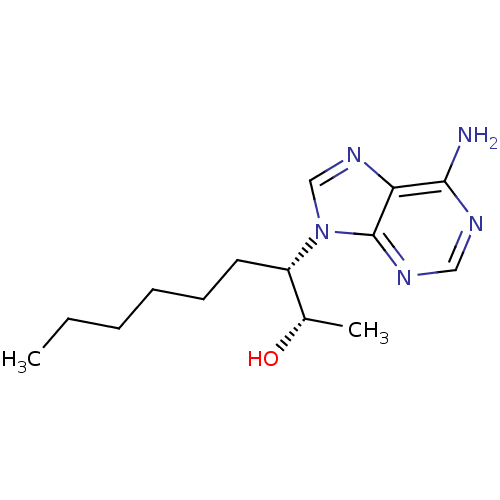
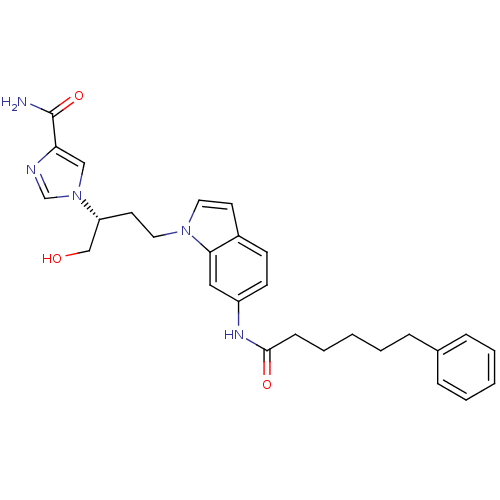
Affinity DataKi: 0.000100nMAssay Description:Inhibition of adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.000100nMAssay Description:Inhibition of adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.00250nMAssay Description:The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminase.Checked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 0.00250nMAssay Description:Binding affinity towards Adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.00250nMAssay Description:Inhibition of adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.00300nMAssay Description:Binding affinity to human erythrocytic ADA assessed as inhibition constant by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.0100nMAssay Description:The compound was tested in vitro for inhibition of human erythrocytic adenosine deaminaseChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 0.0100nMAssay Description:Inhibition of adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.0100nMAssay Description:Binding affinity to ADA (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 0.0100nMAssay Description:Binding affinity towards Adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.0260nMAssay Description:Inhibition of human erythrocytes ADA assessed as equilibrium dissociation constant by measuring reduction in formation of inosine using adenosine as ...More data for this Ligand-Target Pair
Affinity DataKi: 0.0330nM ΔG°: -14.1kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 0.0530nMAssay Description:Inhibition of adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Compound was evaluated for the inhibition of adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Compound was evaluated for the inhibition of adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Inhibition of human erythrocytes ADA assessed as equilibrium dissociation constant by measuring reduction in formation of inosine using adenosine as ...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of human erythrocytes ADA assessed as reduction in formation of inosine using adenosine as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Binding affinity to ADA (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compound was evaluated for the inhibition of adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Inhibition constant (Ki) against Human erythrocyte adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.90nM ΔG°: -11.2kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Inhibition constant (Ki) against Human erythrocyte adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 7.5nM ΔG°: -11.0kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 7.70nM ΔG°: -10.9kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 7.70nM ΔG°: -10.9kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 7.70nM ΔG°: -10.9kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 7.70nMAssay Description:Binding affinity towards adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 9.80nM ΔG°: -10.8kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: >10nMAssay Description:Inhibition of human erythrocytes ADA assessed as reduction in formation of inosine using adenosine as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 11nM ΔG°: -10.7kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 11nM ΔG°: -10.7kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 12nM ΔG°: -10.7kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 13nM ΔG°: -10.6kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 13nM ΔG°: -10.6kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 13nM ΔG°: -10.6kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of human erythrocytes ADA assessed as reduction in formation of inosine using adenosine as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 16nM ΔG°: -10.5kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 17nM ΔG°: -10.5kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 17nM ΔG°: -10.5kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 24nM ΔG°: -10.3kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 26nM ΔG°: -10.2kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 30nM ΔG°: -10.2kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 34nM ΔG°: -10.1kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 37nM ΔG°: -10.0kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 38nM ΔG°: -10.0kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Inhibition of adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 55nM ΔG°: -9.80kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 57nM ΔG°: -9.78kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Compound was evaluated for the inhibition of adenosine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 91nM ΔG°: -9.50kcal/molepH: 7.4 T: 2°CAssay Description:The reaction velocity was measured by change in absorbance at 265nm (A265) resulting from the deamination of adenosine. The reaction was started by a...More data for this Ligand-Target Pair

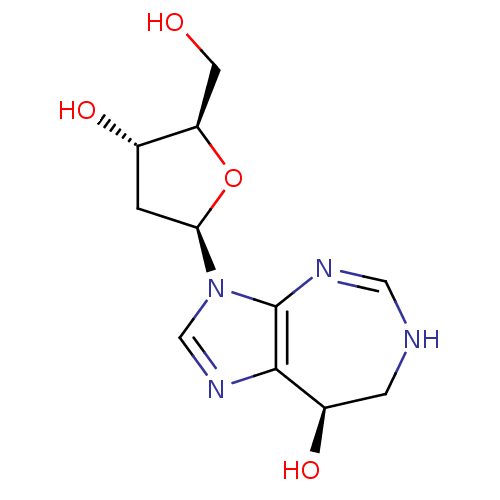

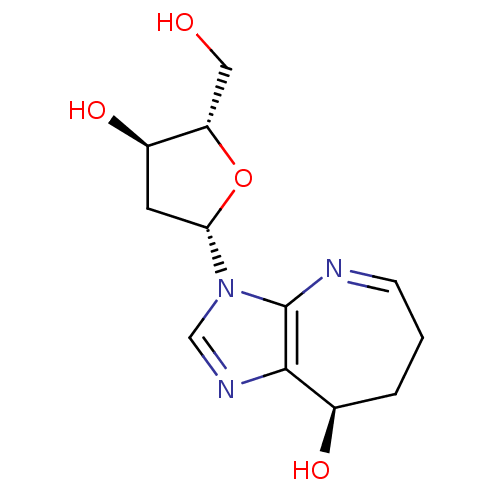
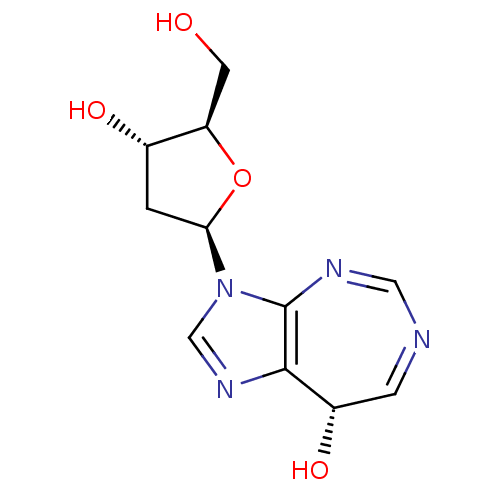

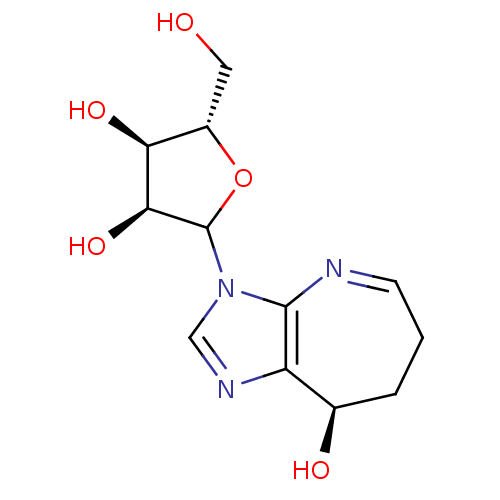
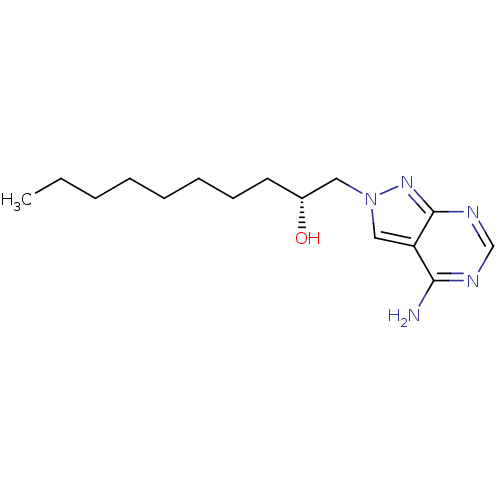
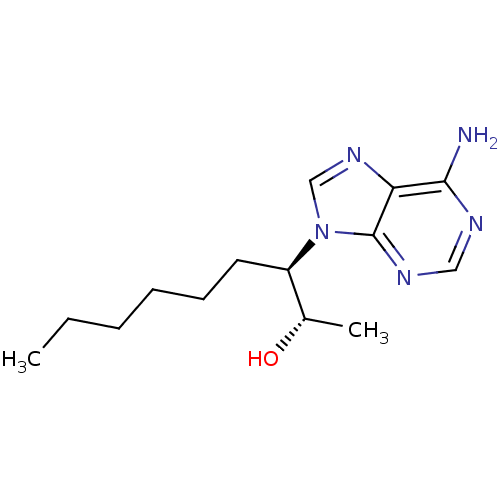
 3D Structure (crystal)
3D Structure (crystal)