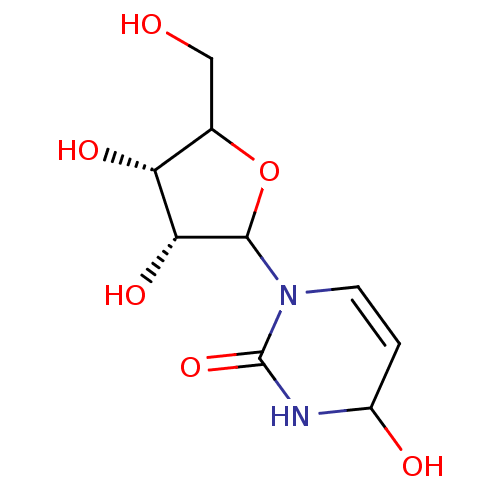
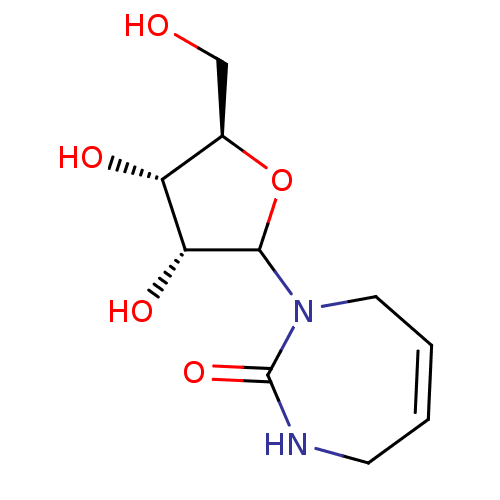
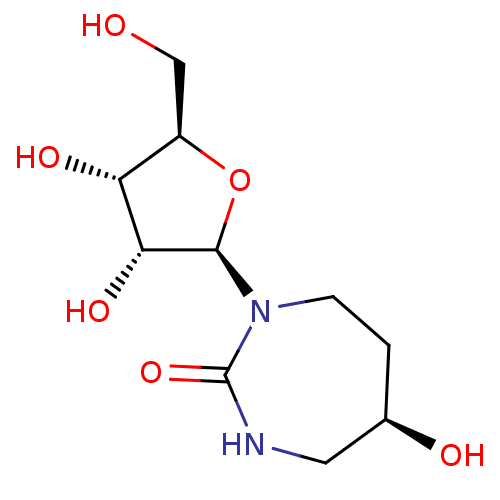
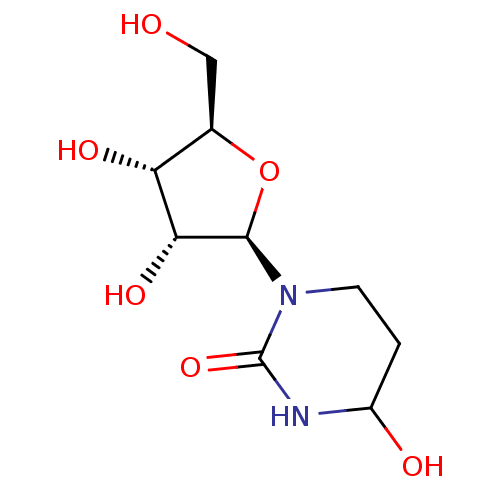
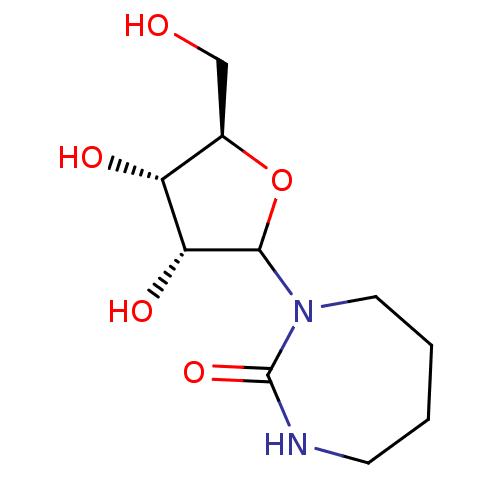
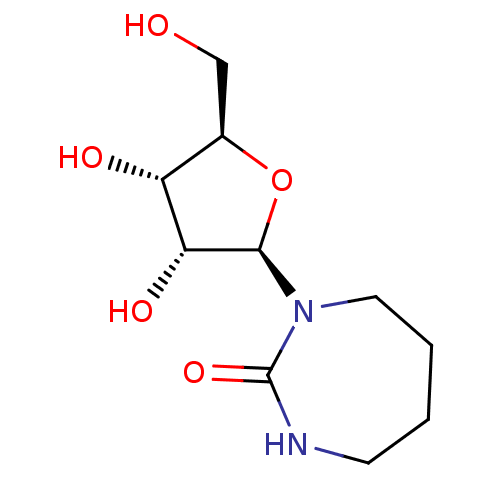
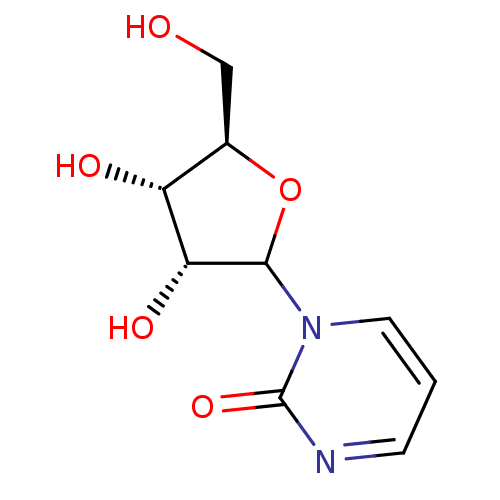
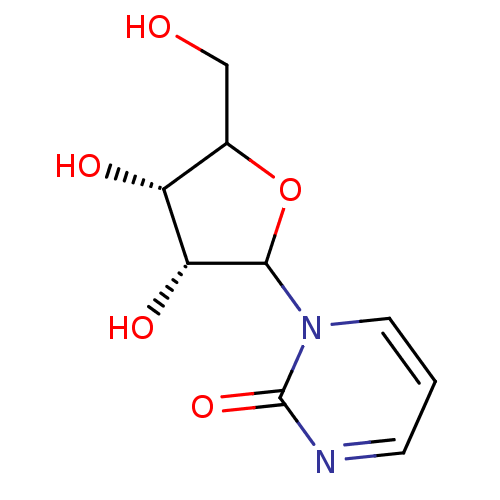
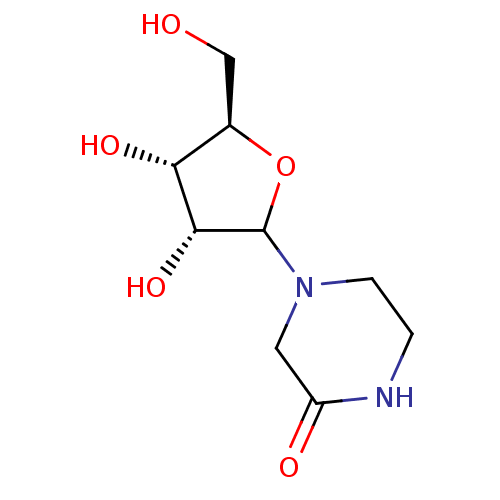
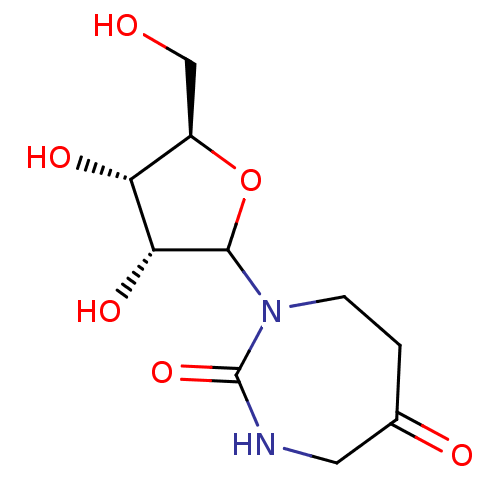
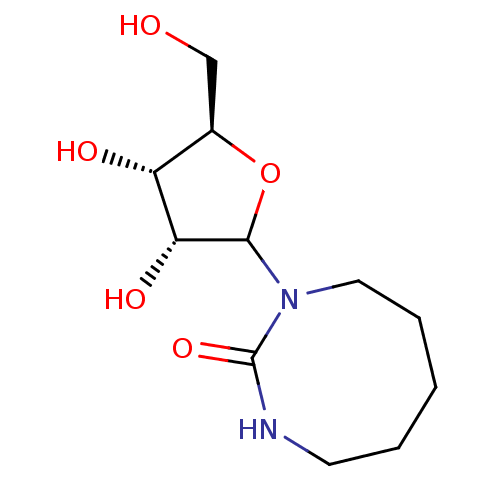
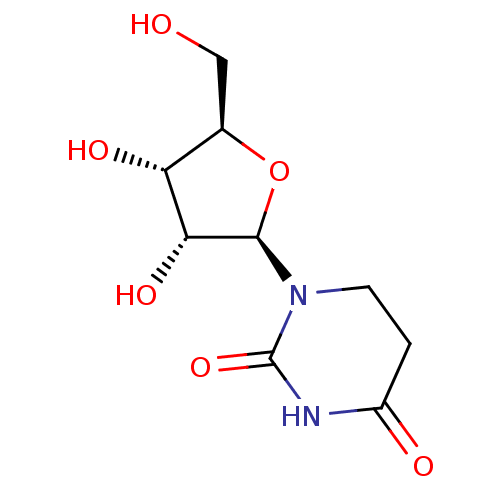
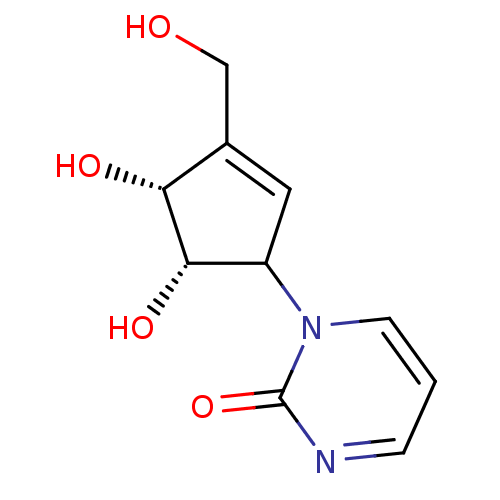
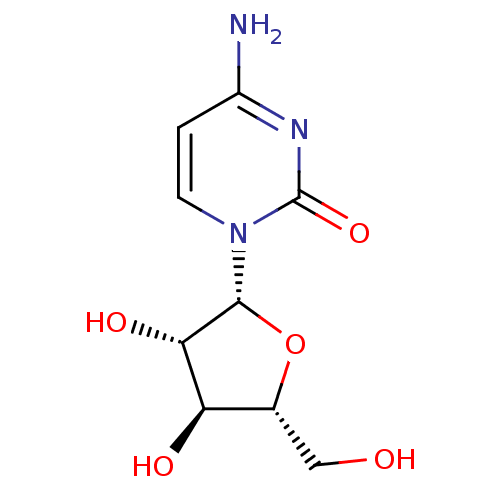
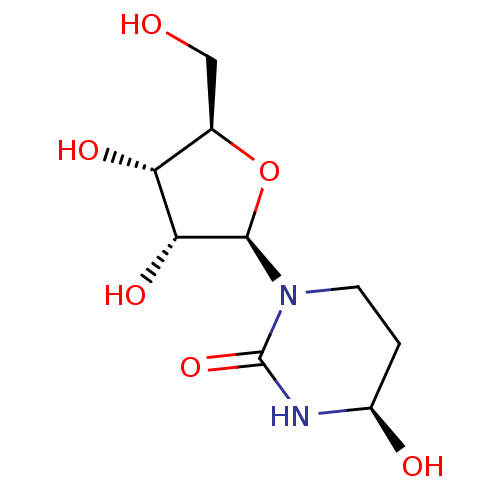
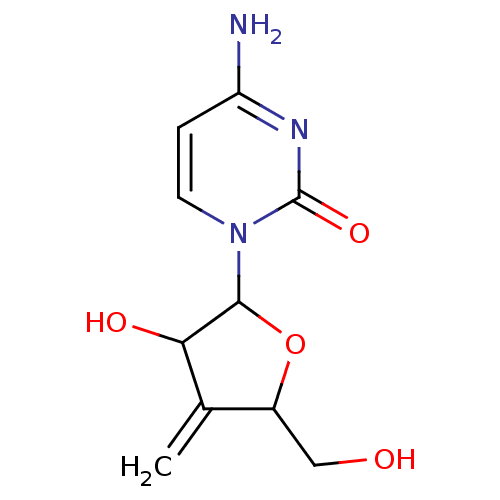
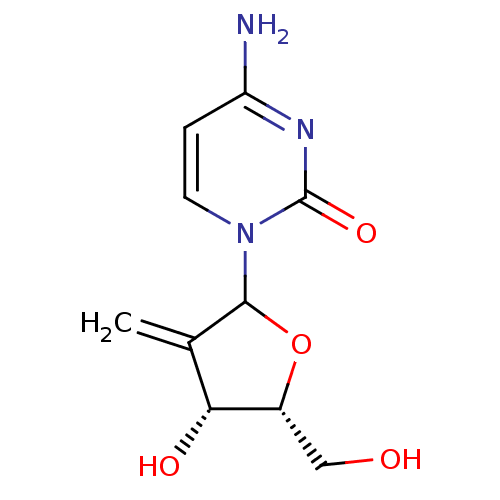
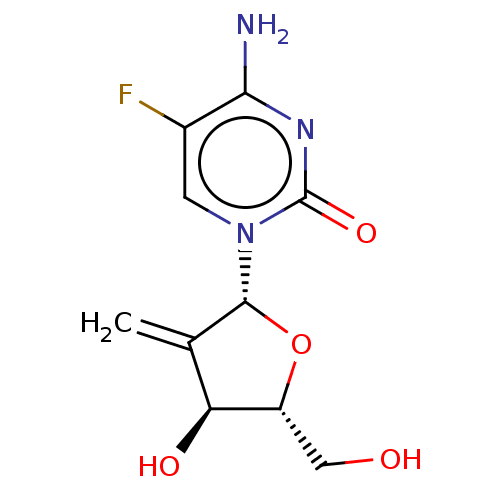
Affinity DataKi: 0.00120nMAssay Description:Compound was tested for its binding affinity against cytidine deaminase.More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Binding affinity for human cytidine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Binding affinity against cytidine deaminase of human liverMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Cytidine Deaminase Inhibition in human liverChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Binding affinity against cytidine deaminase of human liverMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Cytidine Deaminase Inhibition in human liverChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Cytidine Deaminase Inhibition in human liverChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Binding affinity against cytidine deaminase of human liverMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Binding affinity for human cytidine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 400nMAssay Description:Binding affinity for human cytidine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 900nMAssay Description:Cytidine Deaminase Inhibition in human liverChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Binding affinity for human cytidine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+3nMAssay Description:Apparent Ki (binding affinity) was calculated for the compound against cytidine deaminase.More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Binding affinity against cytidine deaminase of human liverMore data for this Ligand-Target Pair
Affinity DataKi: 9.00E+3nMAssay Description:Cytidine Deaminase Inhibition in human liverChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Binding affinity against cytidine deaminase of human liverMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+4nMAssay Description:Compound was tested for its binding affinity against cytidine deaminase.More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+4nMAssay Description:Inhibition of human cytidine deaminase by spectrophotometricallyMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+5nMAssay Description:Compound was tested for its binding affinity against cytidine deaminase.More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+5nMAssay Description:Inhibitory constant was measured on cytidine/deoxycytidine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+5nMAssay Description:Binding affinity against cytidine deaminase of human liverMore data for this Ligand-Target Pair
Affinity DataKi: 4.40E+5nMAssay Description:Inhibition of human cytidine deaminase by spectrophotometricallyMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+6nMAssay Description:Inhibitory constant was measured on cytidine/deoxycytidine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 7.69E+6nMAssay Description:Inhibitory constant was measured on cytidine/deoxycytidine deaminaseMore data for this Ligand-Target Pair
Affinity DataKi: 2.51E+7nMAssay Description:Inhibitory constant was measured on cytidine/deoxycytidine deaminaseMore data for this Ligand-Target Pair