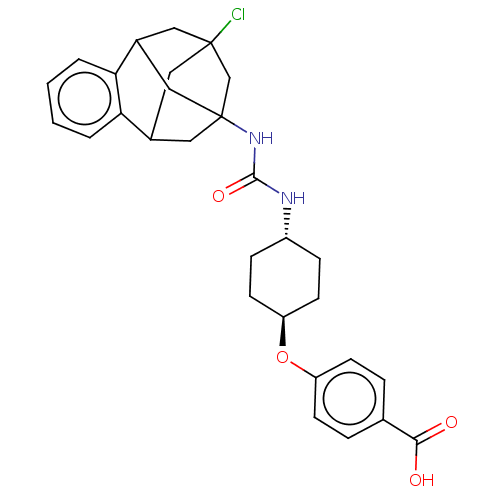
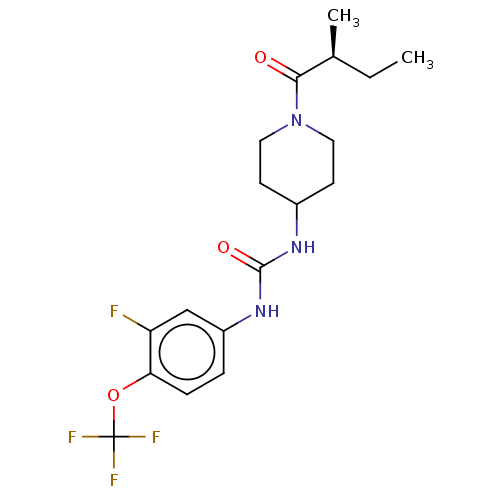
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
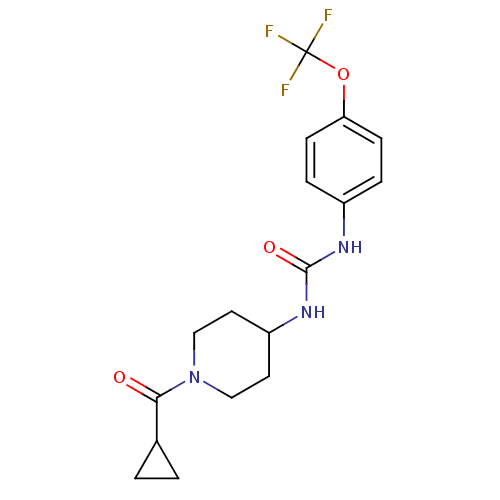
Affinity DataIC50: 0.0270nMAssay Description:Inhibition of recombinant human sEH using MNPC as substrate by fluorescence-based assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
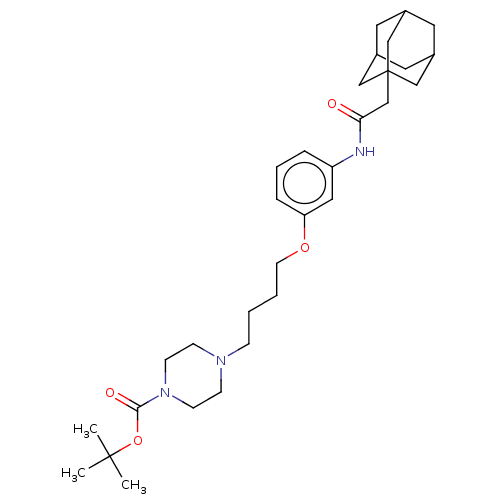
Affinity DataIC50: 0.0270nMAssay Description:Inhibition of recombinant human soluble epoxide hydrolaseMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
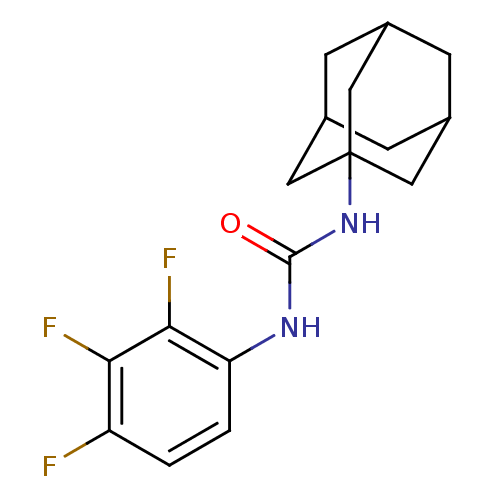
Affinity DataIC50: 0.0280nMAssay Description:Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
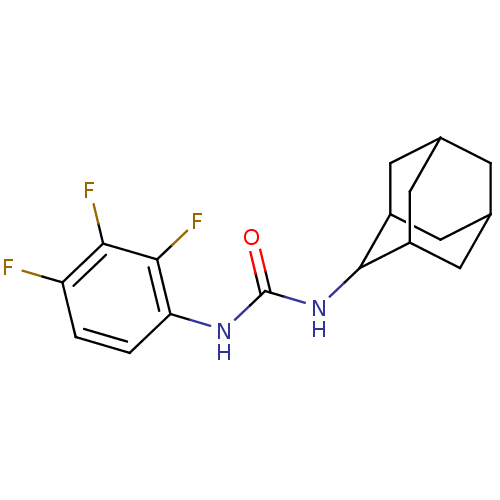
Affinity DataIC50: 0.0280nMAssay Description:Inhibition of human recombinant sEH using 14,15-epoxy-5Z,8Z,11Z-eicosatrienoic acid as substrate assessed as formation of 14,15-dihydroxy-5Z,8Z,11Zei...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of sEH (unknown origin)More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.0820nMAssay Description:Inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of soluble EH in human HepG2 cells by cellular assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.122nMAssay Description:Compounds were tested in a biochemical screening assay using recombinant sEH purified from Sf9 insect cells and an artificial substrate, (3-phenyl-ox...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.125nMAssay Description:Inhibition of human recombinant sEH expressed in baculovirus infected insect Sf9 cells using cyano(6-methoxynaphthalen-2-yl)methyl trans-[(3-phenylox...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.168nMAssay Description:Compounds were tested in a biochemical screening assay using recombinant sEH purified from Sf9 insect cells and an artificial substrate, (3-phenyl-ox...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.25nMAssay Description:Inhibition of recombinant human sEH using PHOME as substrate measured after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of sEH (unknown origin)More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of sEH (unknown origin)More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human recombinant sEH using cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl]carbonate after 20 to 45 mins by ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase by fluorescence assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase by fluorescence assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using Nonfluorescent Cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl] carbonate as subs...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using Nonfluorescent Cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl] carbonate as subs...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using Nonfluorescent Cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl] carbonate as subs...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using Nonfluorescent Cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl] carbonate as subs...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using Nonfluorescent Cyano(6-methoxy-naphthalen-2-yl)methyl trans-[(3-phenyloxiran-2-yl)methyl] carbonate as subs...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measured every 30 sec for 10 ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measured every 30 sec for 10 ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using MNPC as substrate by fluorescence-based assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using MNPC as substrate by fluorescence-based assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of soluble epoxide hydrolase (unknown origin)More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of soluble epoxide hydrolase (unknown origin)More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant soluble epoxide hydrolase using cyano(6-methoxy-naphthalen-2-yl)methyl-trans-[(3-phenyloxiran-2-yl)methyl]carbonate a...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human liver soluble epoxide hydrolase expressed in baculovirus infected Sf21 cells using NEPC as substrate by fluorescence ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human liver soluble epoxide hydrolase expressed in baculovirus infected Sf21 cells using NEPC as substrate by fluorescence ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human liver soluble epoxide hydrolase expressed in baculovirus infected Sf21 cells using NEPC as substrate by fluorescence ...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human soluble epoxide hydrolase using cyano(2methoxy naphthalen-6-yl)methyl trans-(3-phenyloxyran-2-yl)methylcarbonate as substrate pre...More data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human soluble epoxide hydrolase by kinetic fluorescent assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant sEH using PHOME as substrate measured after 10 mins by fluorescent based assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant sEH using PHOME as substrate measured after 10 mins by fluorescent based assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant sEH using CMNPC as substrate incubated for 5 mins by fluorescent based assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of recombinant human sEH using CMNPC as substrate incubated for 10 mins and measured by fluorescence assayMore data for this Ligand-Target Pair
TargetBifunctional epoxide hydrolase 2(Homo sapiens (Human))
University Of California Davis
Curated by ChEMBL
University Of California Davis
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human recombinant sEHMore data for this Ligand-Target Pair

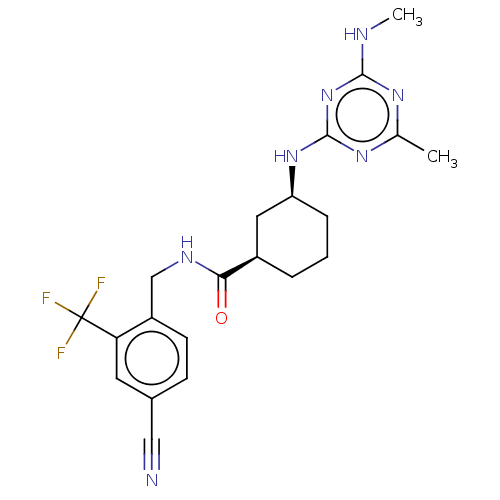
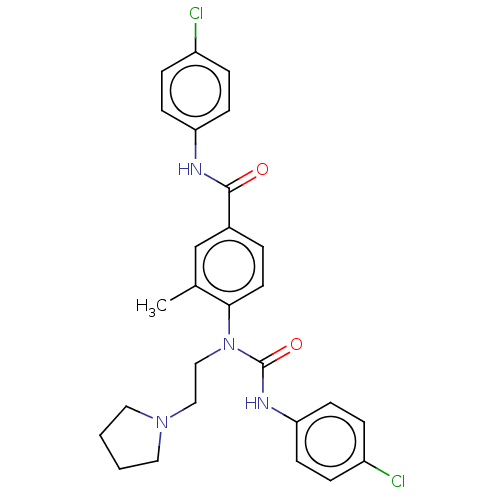
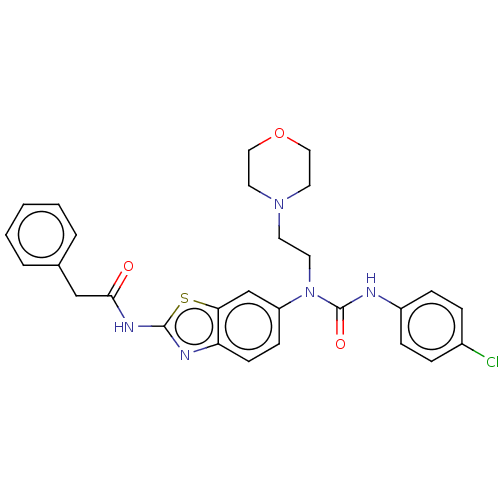
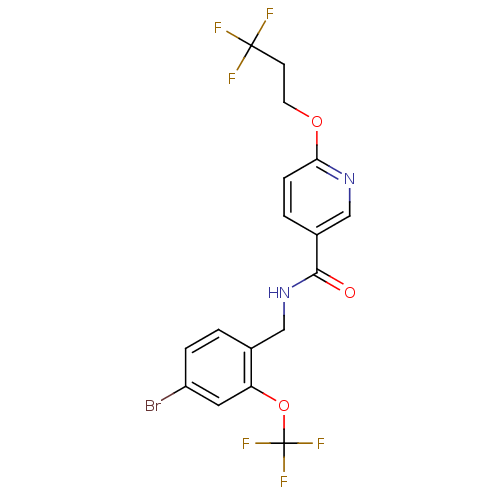
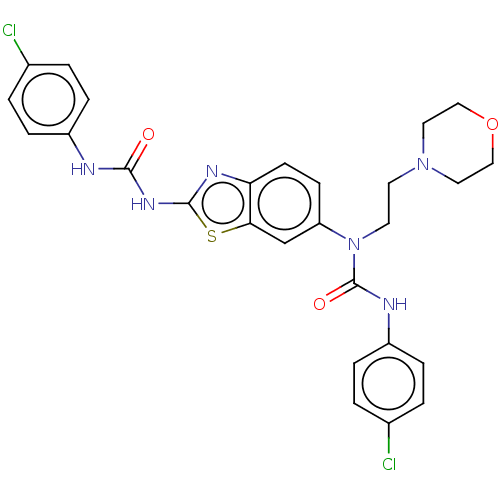
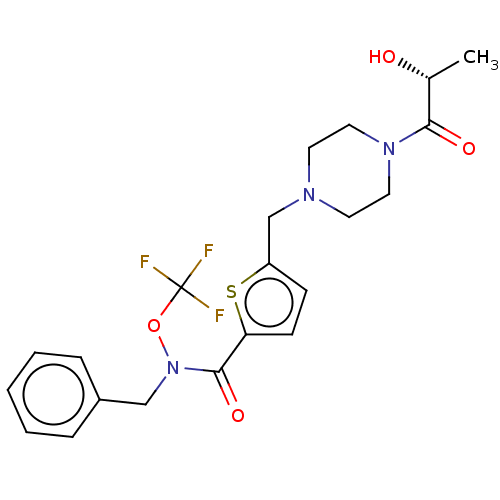
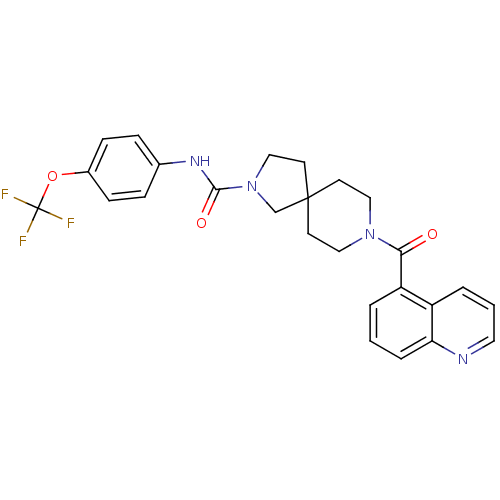



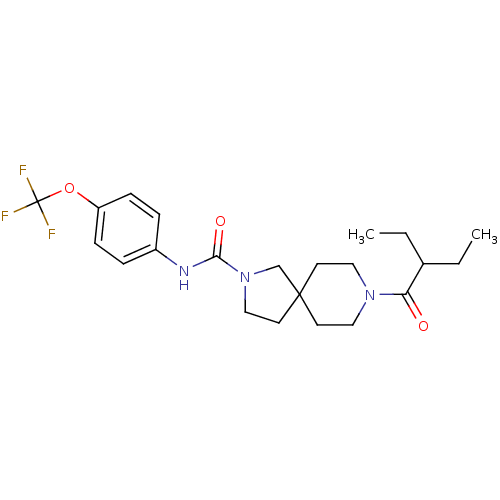
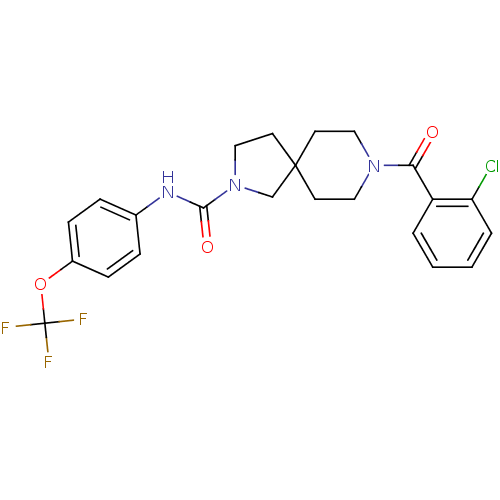
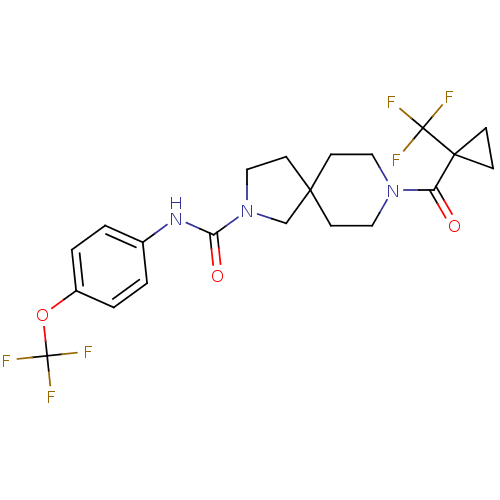
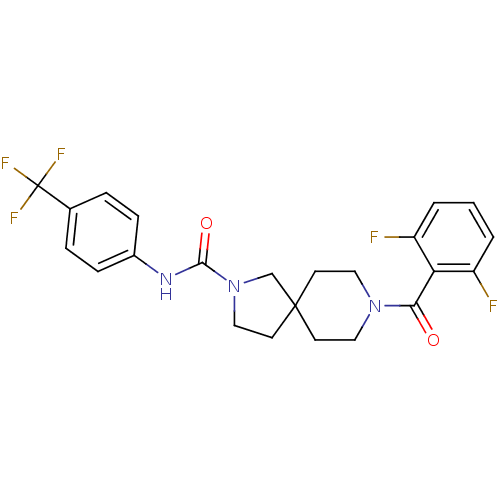
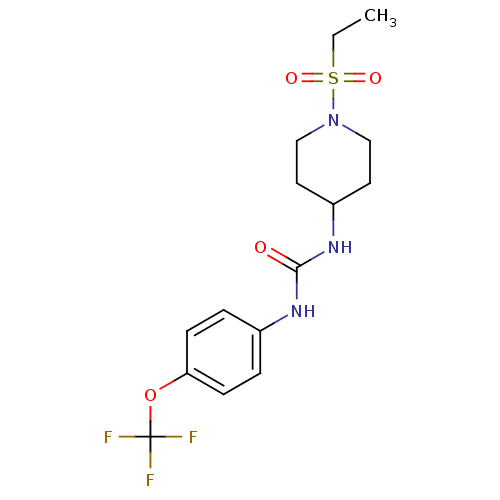
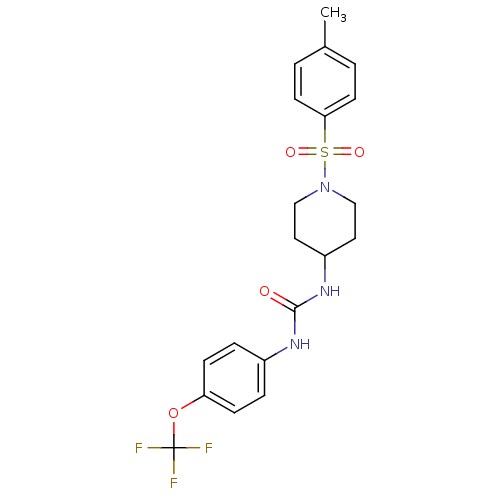
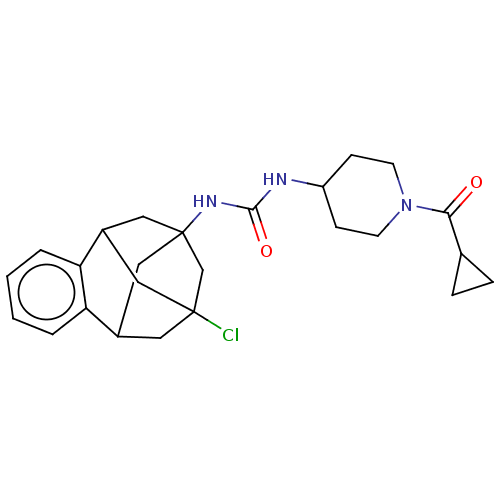
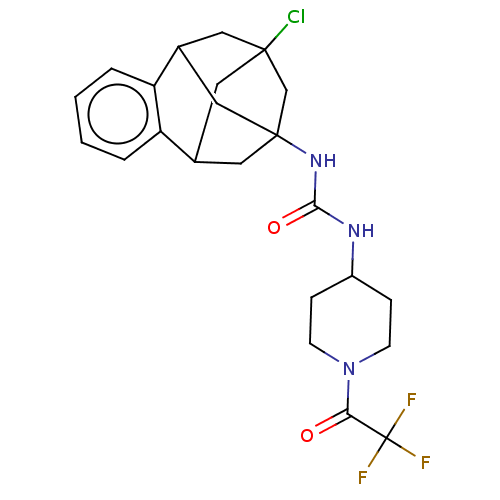
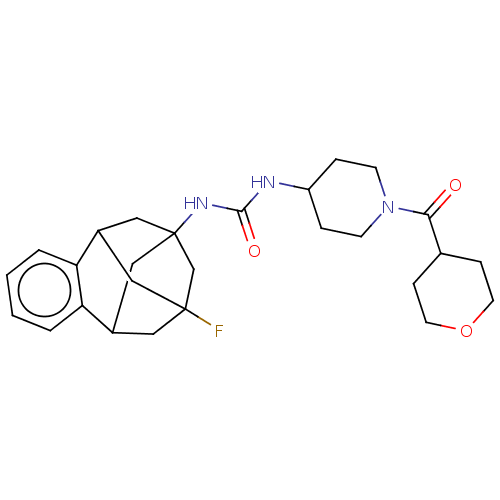
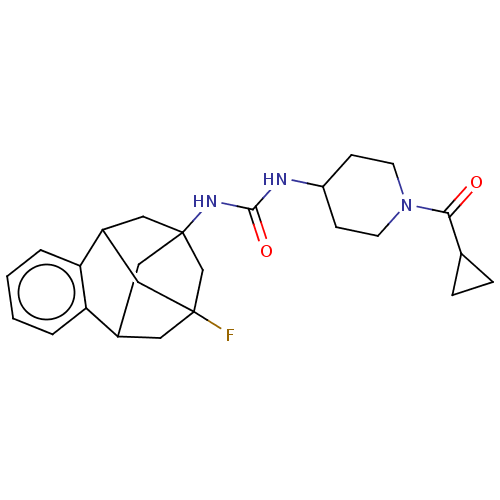
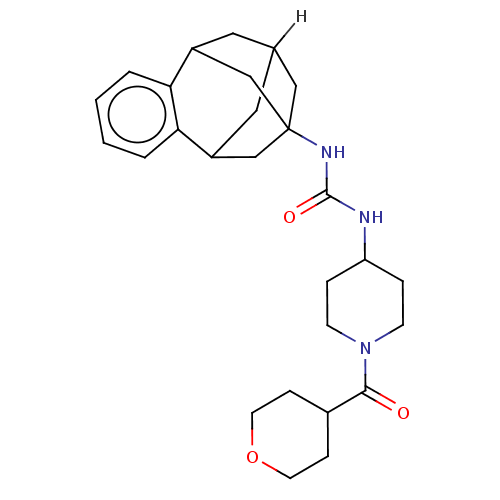
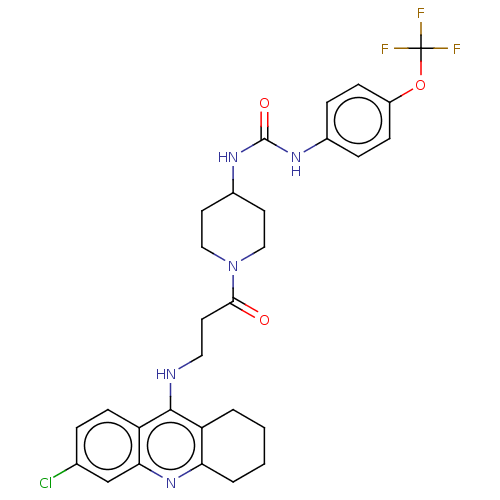
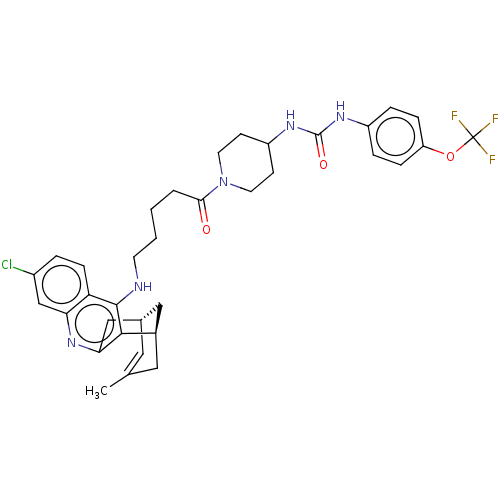
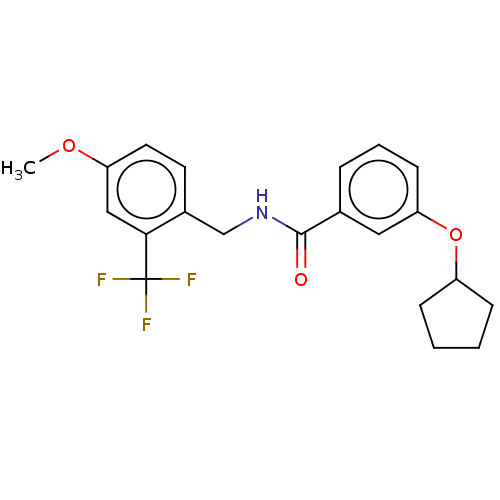
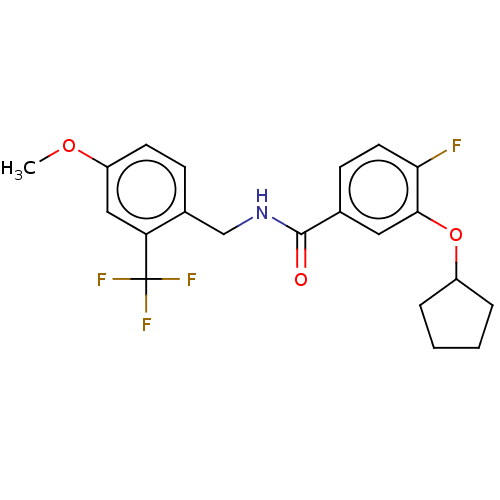
 3D Structure (crystal)
3D Structure (crystal)