TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 430nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 540nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 1.50E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase F(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Inhibition of LAR (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectrophot...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 2.40E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 2.40E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 2.60E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 2.70E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 3.70E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 3.90E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 3.90E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 4.70E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 4.80E+3nMAssay Description:Inhibition of TC-PTP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectrop...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 5.60E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 5.90E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectroph...More data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 6.20E+3nMAssay Description:Inhibition of LMW-PTP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectro...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 6.80E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
Affinity DataKi: 6.90E+3nMAssay Description:Inhibition of MKP5 (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 9.60E+3nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 1.40E+4nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 1.50E+4nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Inhibition of STEP (unknown origin) using pNPP as substrate preincubated for 5 mins followed by substrate addition measured for 20 mins by spectropho...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKd: 3.14E+4nMAssay Description:Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKd: 2.72E+4nMAssay Description:Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 5(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataKd: 7.04E+4nMAssay Description:Binding affinity to 6His-tagged STEP phosphatase domain (258 to 539 residues) (unknown origin) expressed in Escherichia coli BL21 Gold (DE3) after 90...More data for this Ligand-Target Pair

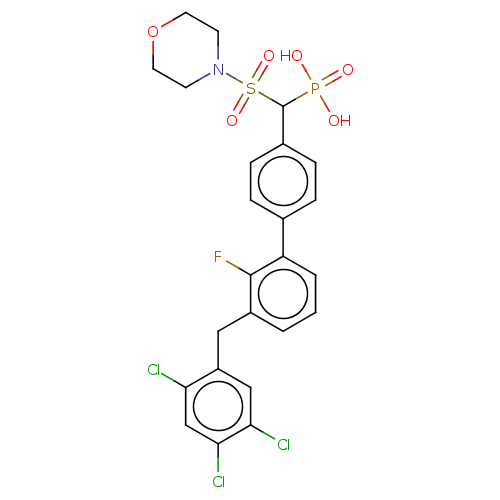
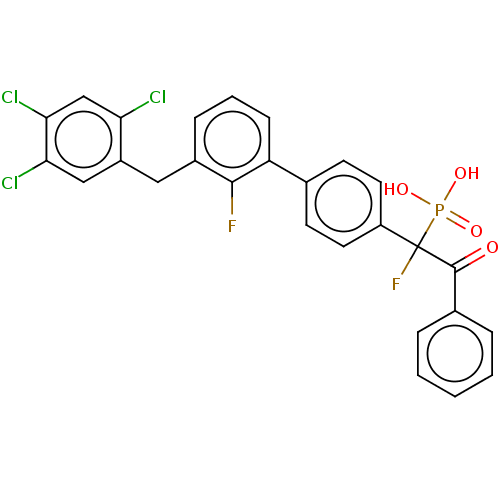
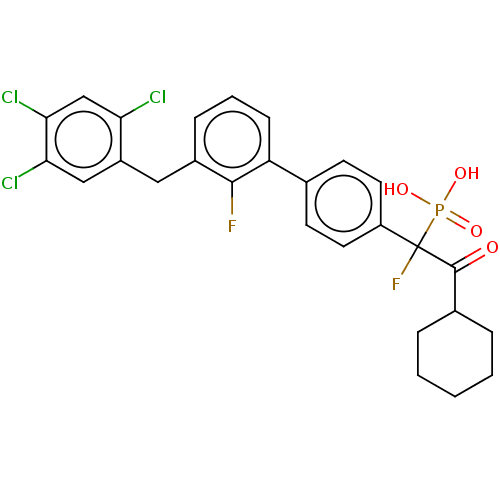
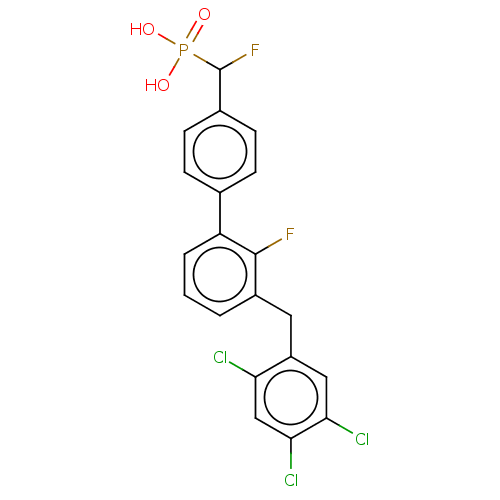
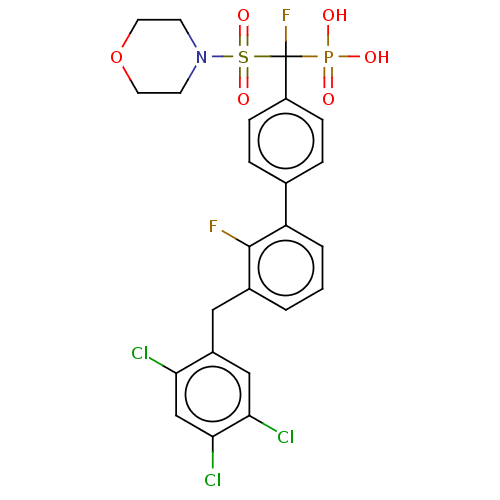
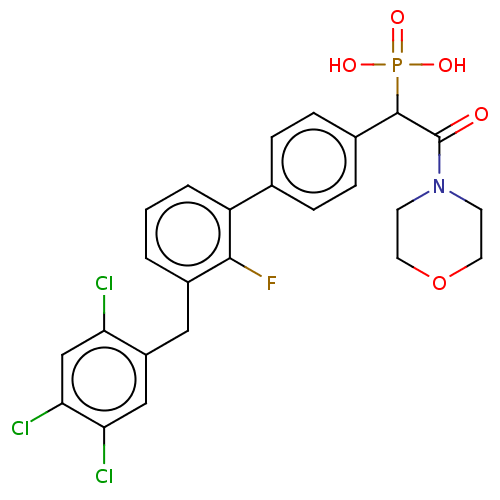
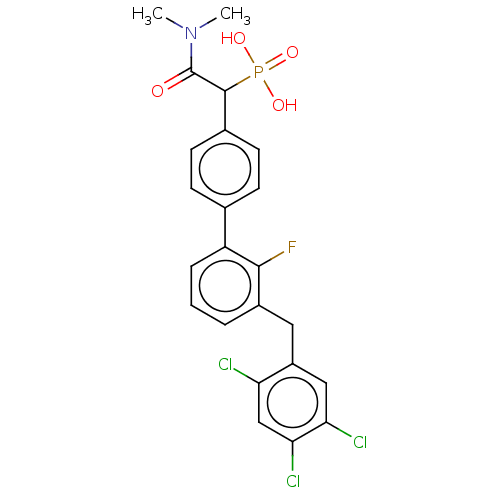
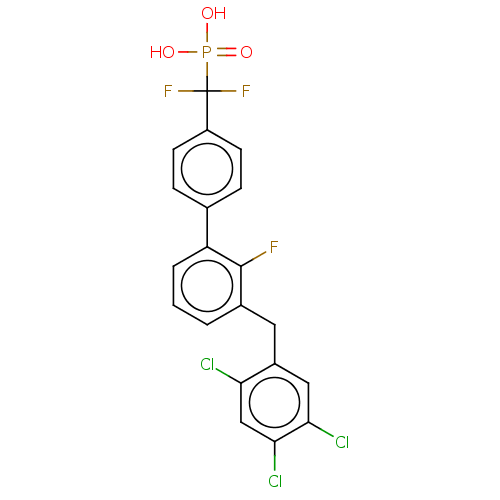
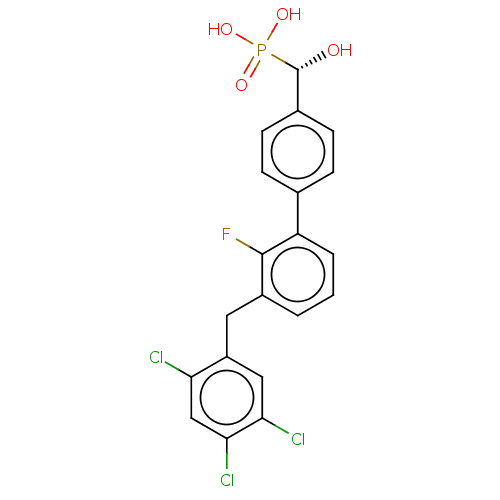

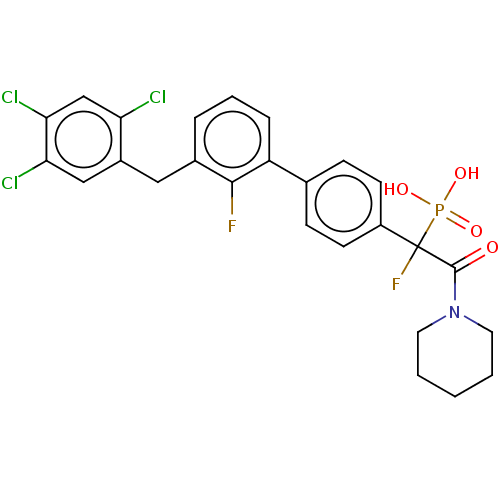
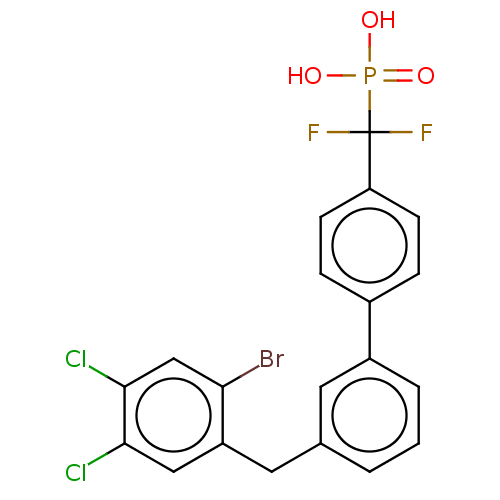
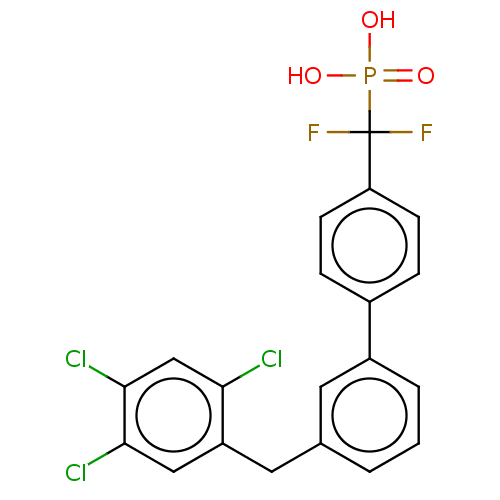
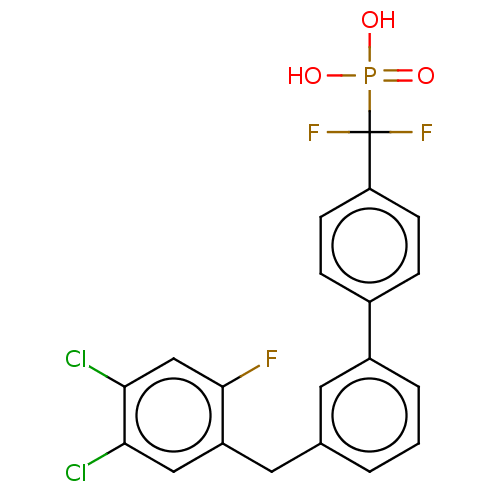
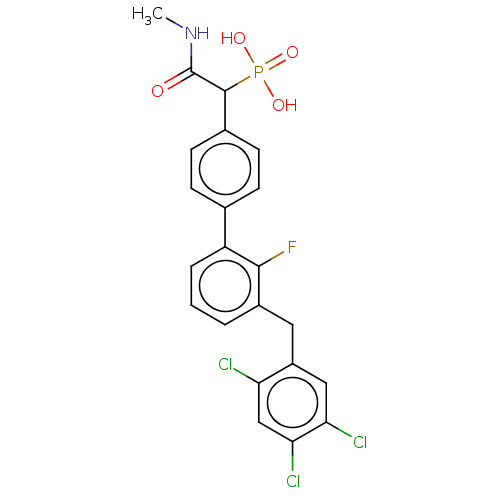
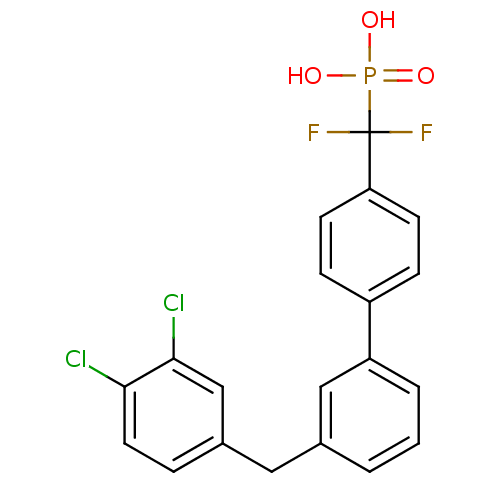
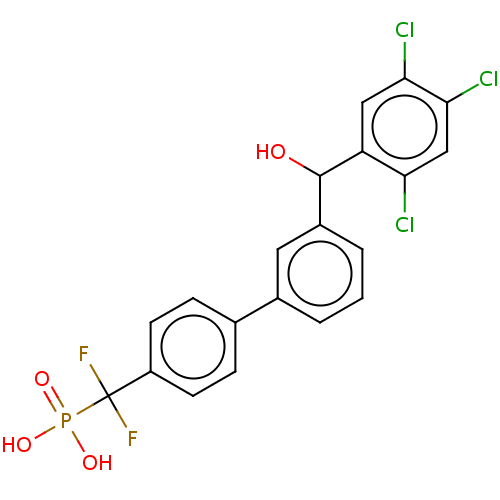
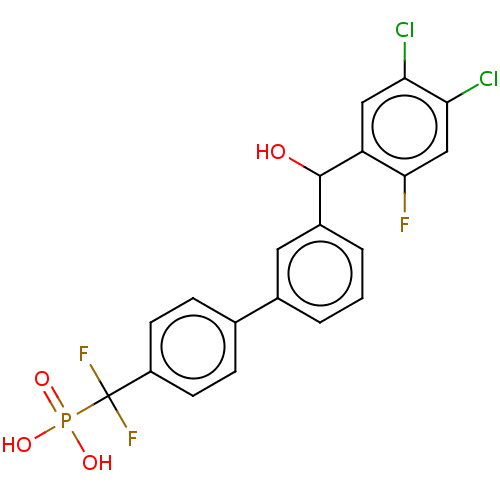
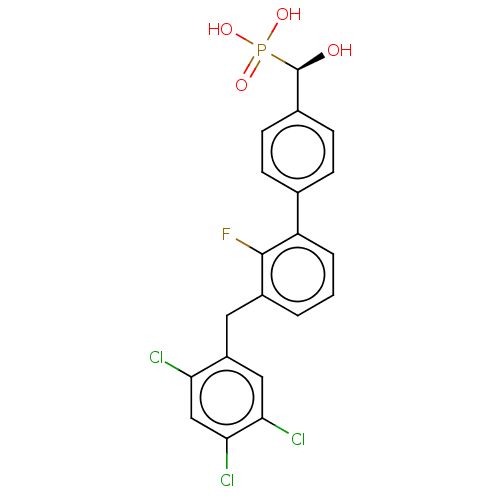
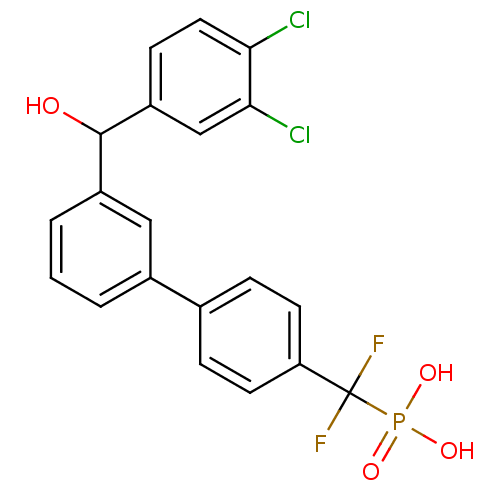
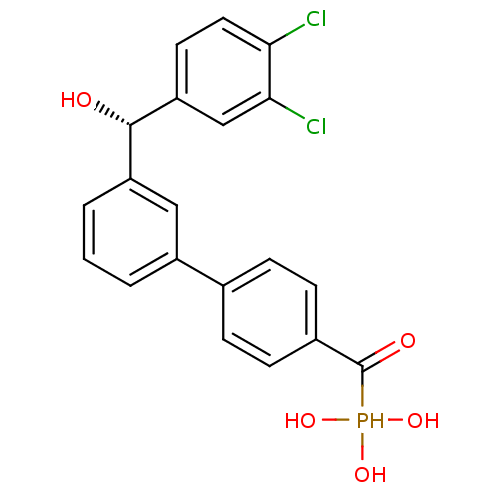


 3D Structure (crystal)
3D Structure (crystal)