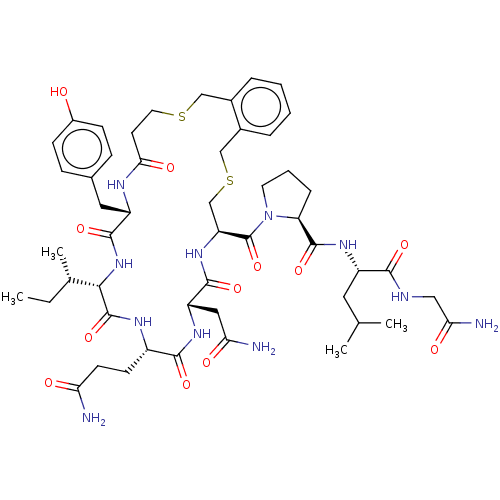
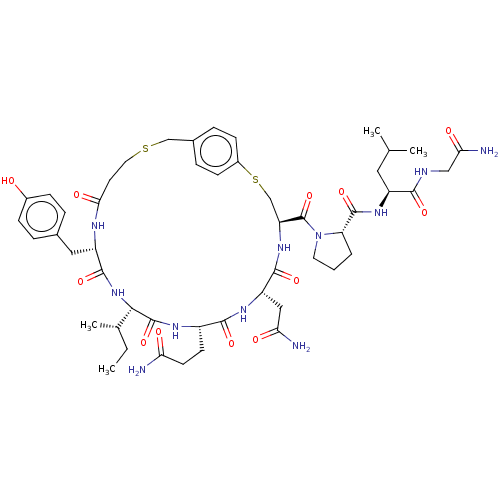
Affinity DataKi: 1.20nMAssay Description:Displacement of [3H]OT from human mammary gland oxytocin receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H]AVP from human liver vasopressin V1a receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Displacement of [3H]AVP from human vasopressin V1b receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Displacement of [3H]OT from human mammary gland oxytocin receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of [3H]AVP from human liver vasopressin V1a receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Displacement of [3H]AVP from human liver vasopressin V1a receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Displacement of [3H]AVP from human kidney vasopressin V2 receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Displacement of [3H]OT from human mammary gland oxytocin receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 69nMAssay Description:Displacement of [3H]AVP from human liver vasopressin V1a receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 142nMAssay Description:Displacement of [3H]OT from human mammary gland oxytocin receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 166nMAssay Description:Displacement of [3H]OT from human mammary gland oxytocin receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Displacement of [3H]OT from human mammary gland oxytocin receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.68E+3nMAssay Description:Displacement of [3H]AVP from human liver vasopressin V1a receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.95E+3nMAssay Description:Displacement of [3H]AVP from human liver vasopressin V1a receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >6.00E+3nMAssay Description:Displacement of [3H]AVP from human kidney vasopressin V2 receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >6.00E+3nMAssay Description:Displacement of [3H]AVP from human kidney vasopressin V2 receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >6.00E+3nMAssay Description:Displacement of [3H]AVP from human kidney vasopressin V2 receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >6.00E+3nMAssay Description:Displacement of [3H]AVP from human kidney vasopressin V2 receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >6.00E+3nMAssay Description:Displacement of [3H]AVP from human vasopressin V1b receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >6.00E+3nMAssay Description:Displacement of [3H]AVP from human vasopressin V1b receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >6.00E+3nMAssay Description:Displacement of [3H]AVP from human vasopressin V1b receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >6.00E+3nMAssay Description:Displacement of [3H]AVP from human vasopressin V1b receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >6.00E+3nMAssay Description:Displacement of [3H]AVP from human kidney vasopressin V2 receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: >6.00E+3nMAssay Description:Displacement of [3H]AVP from human vasopressin V1b receptor expressed in HEK293 cell membrane after 1 hr by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Antagonist activity at human liver vasopressin V1a receptor expressed in CHO cells assessed as inhibition of agonist-induced intracellular calcium fl...More data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Antagonist activity at human liver vasopressin V1a receptor expressed in CHO cells assessed as inhibition of agonist-induced intracellular calcium fl...More data for this Ligand-Target Pair
Affinity DataIC50: 1.72E+3nMAssay Description:Antagonist activity at human mammary gland oxytocin receptor expressed in CHO cells assessed as inhibition of agonist-induced intracellular calcium f...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:Antagonist activity at human liver vasopressin V1a receptor expressed in CHO cells assessed as inhibition of agonist-induced intracellular calcium fl...More data for this Ligand-Target Pair
Affinity DataIC50: 7.86E+3nMAssay Description:Antagonist activity at human liver vasopressin V1a receptor expressed in CHO cells assessed as inhibition of agonist-induced intracellular calcium fl...More data for this Ligand-Target Pair
Affinity DataEC50: 294nMAssay Description:Agonist activity at human mammary gland oxytocin receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 mi...More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human mammary gland oxytocin receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 mi...More data for this Ligand-Target Pair
Affinity DataEC50: 9nMAssay Description:Agonist activity at human liver vasopressin V1a receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 min...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+4nMAssay Description:Agonist activity at human liver vasopressin V1a receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 min...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+4nMAssay Description:Agonist activity at human liver vasopressin V1a receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 min...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+4nMAssay Description:Agonist activity at human liver vasopressin V1a receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 min...More data for this Ligand-Target Pair
Affinity DataEC50: 33nMAssay Description:Agonist activity at human vasopressin V1b receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 mins by F...More data for this Ligand-Target Pair
Affinity DataEC50: 2.97E+3nMAssay Description:Agonist activity at human vasopressin V1b receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 mins by F...More data for this Ligand-Target Pair
Affinity DataEC50: 2.22E+3nMAssay Description:Agonist activity at human vasopressin V1b receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 mins by F...More data for this Ligand-Target Pair
Affinity DataEC50: 9.26E+3nMAssay Description:Agonist activity at human vasopressin V1b receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 mins by F...More data for this Ligand-Target Pair
Affinity DataEC50: 29nMAssay Description:Agonist activity at human kidney vasopressin V2 receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 min...More data for this Ligand-Target Pair
Affinity DataEC50: 3.90E+3nMAssay Description:Agonist activity at human kidney vasopressin V2 receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 min...More data for this Ligand-Target Pair
Affinity DataEC50: 3.67E+3nMAssay Description:Agonist activity at human kidney vasopressin V2 receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 min...More data for this Ligand-Target Pair
Affinity DataEC50: 1.08E+4nMAssay Description:Agonist activity at human kidney vasopressin V2 receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 min...More data for this Ligand-Target Pair
Affinity DataEC50: 1.89E+4nMAssay Description:Agonist activity at human kidney vasopressin V2 receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 min...More data for this Ligand-Target Pair
Affinity DataEC50: 8.30E+3nMAssay Description:Agonist activity at human vasopressin V1b receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 mins by F...More data for this Ligand-Target Pair
Affinity DataEC50: 109nMAssay Description:Agonist activity at human mammary gland oxytocin receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 mi...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+4nMAssay Description:Agonist activity at human liver vasopressin V1a receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 min...More data for this Ligand-Target Pair
Affinity DataEC50: 0.300nMAssay Description:Agonist activity at human mammary gland oxytocin receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 mi...More data for this Ligand-Target Pair
Affinity DataEC50: 34nMAssay Description:Agonist activity at human mammary gland oxytocin receptor expressed in CHO cells assessed as increase in intracellular calcium flux measured for 5 mi...More data for this Ligand-Target Pair