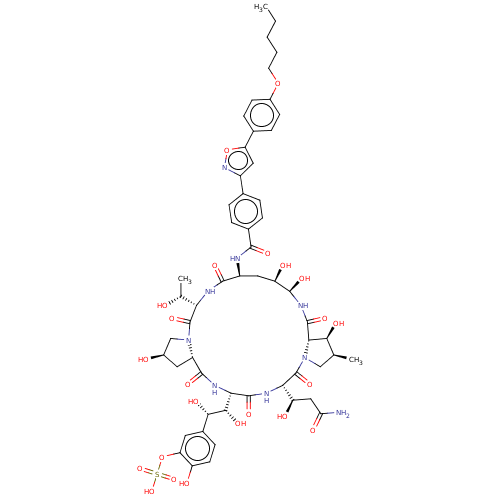
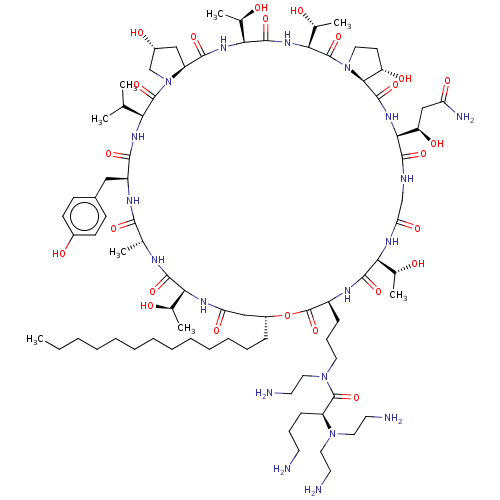
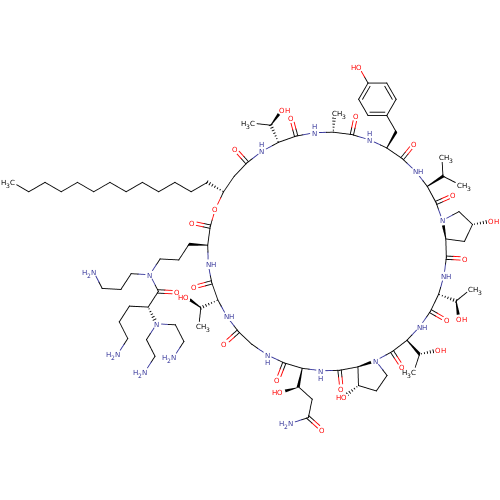
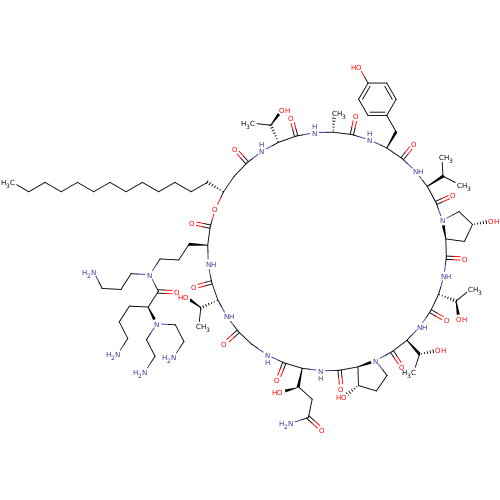
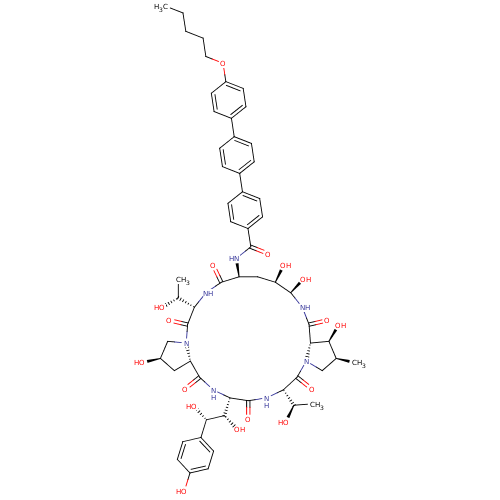
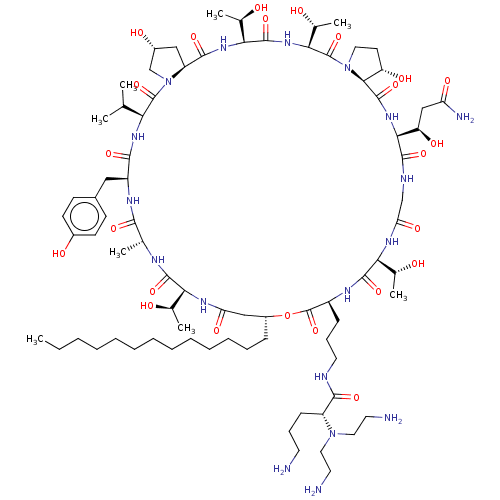
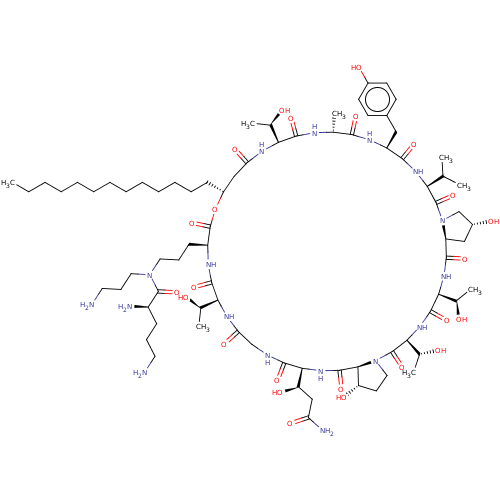
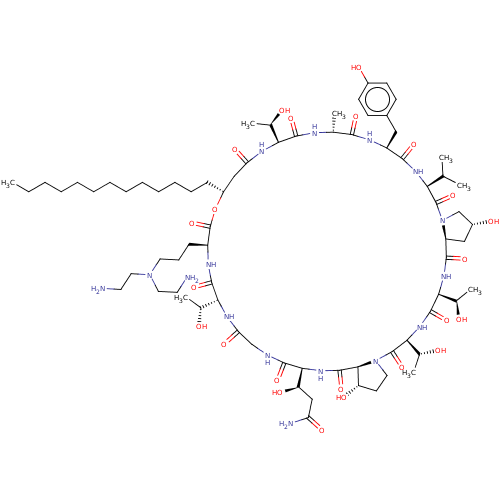
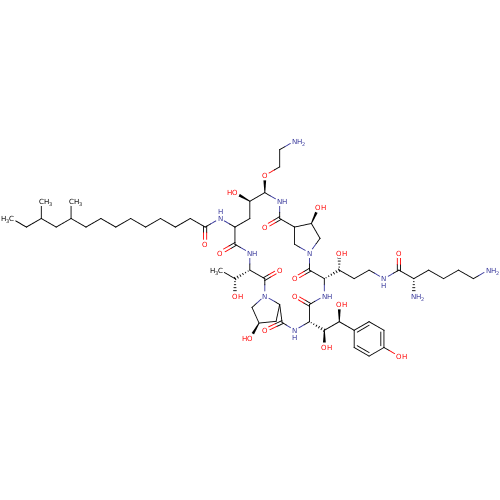
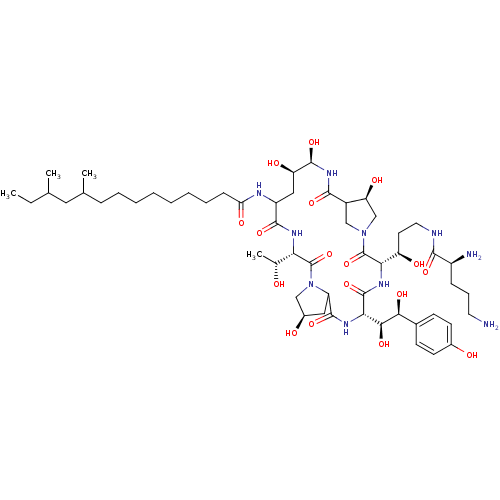
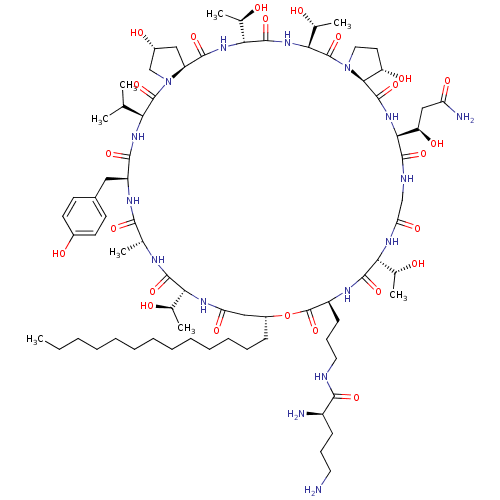
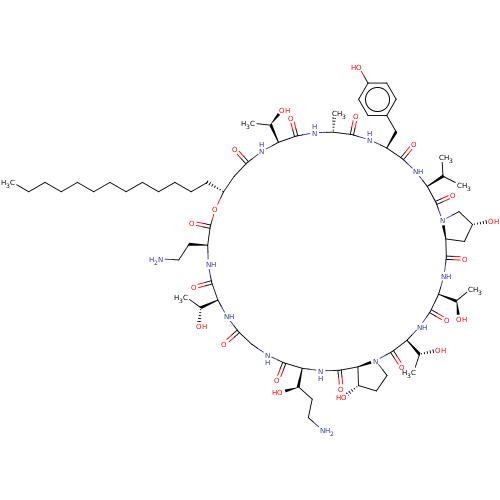
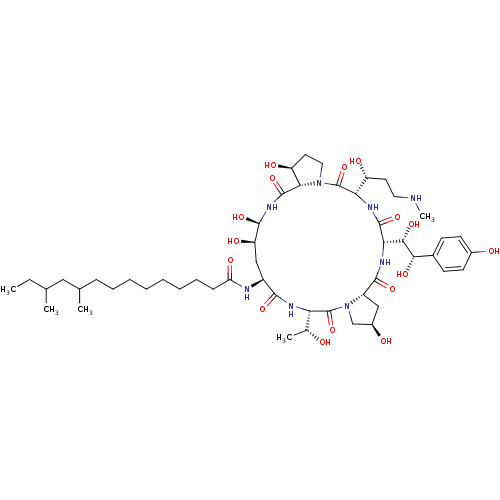
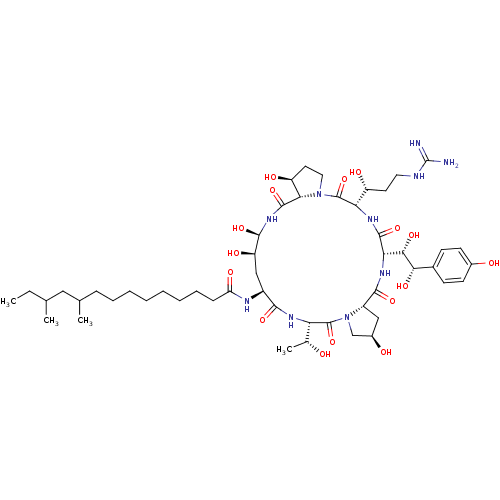
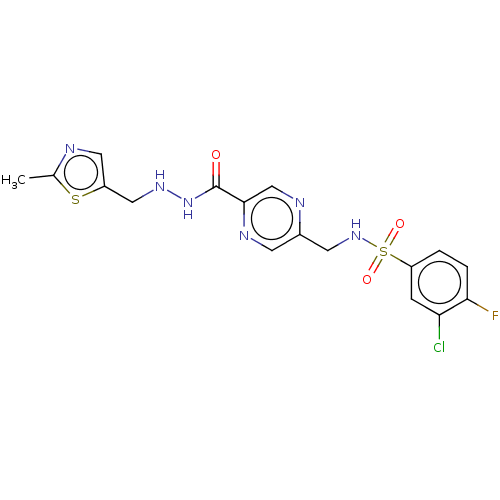
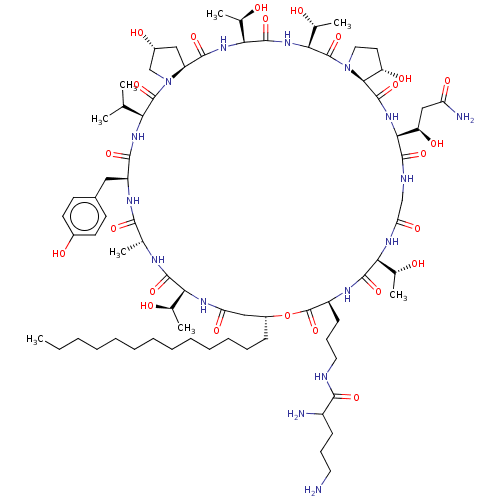
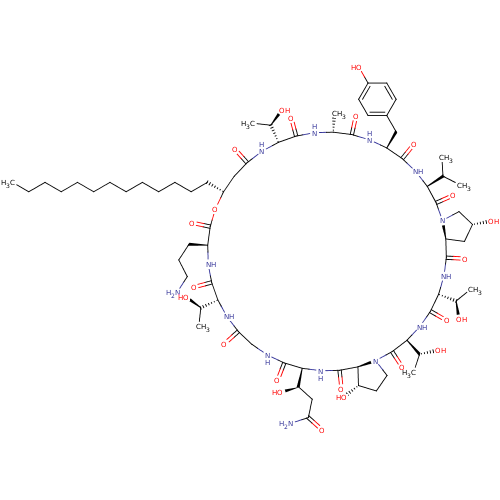
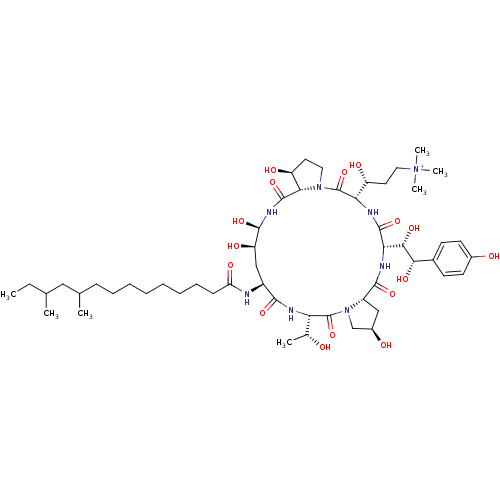
Target1,3-beta-glucan synthase(Candida tropicalis)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 0.117nMAssay Description:Inhibition of Candida tropicalis T3 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at monophasic kineticsMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida tropicalis)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 0.171nMAssay Description:Inhibition of Candida tropicalis T19 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at monophasic kineticsMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:In vitro inhibitory activity against Beta glucan synthaseMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 0.25nMAssay Description:In vitro inhibitory activity against Beta glucan synthaseMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 0.280nMAssay Description:In vitro inhibitory activity against Beta glucan synthaseMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida tropicalis)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 0.281nMAssay Description:Inhibition of Candida tropicalis T3 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at monophasic kineticsMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida tropicalis)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 0.296nMAssay Description:Inhibition of Candida tropicalis T19 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at monophasic kineticsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 90028More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida tropicalis)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 0.306nMAssay Description:Inhibition of Candida tropicalis T3 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at monophasic kineticsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMAssay Description:Inhibition of glucan synthase in Candida albicans 36082 assessed as incorporation of [3H]uridine diphosphoglucoseMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida tropicalis)
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
University Of Medicine And Dentistry Of New Jersey
Curated by ChEMBL
Affinity DataIC50: 0.503nMAssay Description:Inhibition of Candida tropicalis T19 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at monophasic kineticsMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 0.630nMAssay Description:In vitro inhibitory activity against Beta glucan synthaseMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 0.830nMAssay Description:In vitro inhibitory activity against Beta glucan synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.877nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 90028More data for this Ligand-Target Pair
Affinity DataIC50: 0.880nMAssay Description:Inhibition of glucan synthase in Candida albicans 36082 assessed as incorporation of [3H]uridine diphosphoglucoseMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 0.890nMAssay Description:In vitro inhibitory activity against Beta glucan synthaseMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:In vitro inhibitory activity against Beta glucan synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 36082More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of 1,3-beta-glucan synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of glucan synthase in Candida albicans 36082 assessed as incorporation of [3H]uridine diphosphoglucose in presence of 10% serumMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:In vitro inhibitory activity against Beta glucan synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 90028 in presence of 10% human serumMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:In vitro inhibitory activity against Beta glucan synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of 1,3-beta-glucan synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of glucan synthase in Candida albicans 36082 assessed as incorporation of [3H]uridine diphosphoglucose in presence of 20% serumMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicansMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 90028 in presence of 20% human serumMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 36082 in presence of 10% human serumMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of 1,3-beta-glucan synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of 1,3-beta-glucan synthaseMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:In vitro inhibitory activity against Beta glucan synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of 1,3-beta-glucan synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.14nMAssay Description:Inhibition of glucan synthase in Candida albicans 36082 assessed as incorporation of [3H]uridine diphosphoglucose in presence of 10% serumMore data for this Ligand-Target Pair
Affinity DataIC50: 4.14nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 90028 in presence of 10% human serumMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicansMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Tested for inhibition of Beta-1,3-glucan synthase using a crude membrane of Candida albicans (MY 1208).More data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 36082 in presence of 20% human serumMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of 1,3-beta-glucan synthaseMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicansMore data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:Inhibition of glucan synthase in Candida albicans 36082 assessed as incorporation of [3H]uridine diphosphoglucose in presence of 50% serumMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 90028 in presence of 50% human serumMore data for this Ligand-Target Pair
Affinity DataIC50: 6.80nMAssay Description:Inhibition of FKS1 from Candida krusei 98 isolateMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Tested for inhibition of Beta-1,3-glucan synthase using a crude membrane of Candida albicans (MY 1208).More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Tested for inhibition of Beta-1,3-glucan synthase using a crude membrane of Candida albicans (MY 1208).More data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Negative allosteric modulation of human GluN2A receptor expressed in HEK cells assessed as reduction in 3 uM glycine/glutamate-induced intracellular ...More data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of 1,3-beta-D-glucan synthase (GS) from candida albicansMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 8.5nMAssay Description:In vitro inhibitory activity against Beta glucan synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 8.5nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 36082More data for this Ligand-Target Pair
Affinity DataIC50: 8.70nMAssay Description:Inhibition of glucan synthase in Candida albicans ATCC 36082 in presence of 50% human serumMore data for this Ligand-Target Pair
Target1,3-beta-glucan synthase(Candida albicans (Yeast))
Nippon Roche Research Center
Curated by ChEMBL
Nippon Roche Research Center
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Tested for inhibition of Beta-1,3-glucan synthase using a crude membrane of Candida albicans (MY 1208).More data for this Ligand-Target Pair