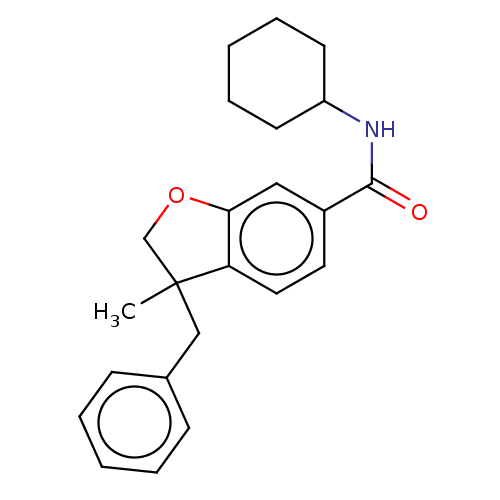
Affinity DataKi: 1.80nMpH: 7.4Assay Description:Cell membrane homogenates (15 μg protein) were incubated for 120 min at 37° C. with 0.8 nM [3H]WIN 55212-2 (the reference standard [Munro, 1993 ...More data for this Ligand-Target Pair
Affinity DataKi: 3.40nMpH: 7.4Assay Description:Cell membrane homogenates (25 μg protein) were incubated for 120 min at 37° C. with 0.5 nM [3H]CP 55940 (the reference standard [Rinaldi-Carmona...More data for this Ligand-Target Pair
Affinity DataKi: 422nMpH: 7.4Assay Description:Cell membrane homogenates (15 μg protein) were incubated for 120 min at 37° C. with 0.8 nM [3H]WIN 55212-2 (the reference standard [Munro, 1993 ...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMpH: 7.4Assay Description:Cell membrane homogenates (25 μg protein) were incubated for 120 min at 37° C. with 0.5 nM [3H]CP 55940 (the reference standard [Rinaldi-Carmona...More data for this Ligand-Target Pair
Affinity DataEC50: 9nMAssay Description:Functional activity was evaluated using GTPγ[35S] assay in CHO membrane extracts expressing recombinant hCB1 (human CB1) receptors or hCB2 (huma...More data for this Ligand-Target Pair
Affinity DataEC50: 128nMAssay Description:Functional activity was evaluated using GTPγ[35S] assay in CHO membrane extracts expressing recombinant hCB1 (human CB1) receptors or hCB2 (huma...More data for this Ligand-Target Pair
Affinity DataEC50: 6.5nMAssay Description:Functional activity was evaluated using GTPγ[35S] assay in CHO membrane extracts expressing recombinant hCB1 (human CB1) receptors or hCB2 (huma...More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:The compound of Example 3 was tested for agonist activity at the rat CB1 (rCB1) and rCB2 receptors, at eight concentrations, in duplicate: 10, 3, 1, ...More data for this Ligand-Target Pair
Affinity DataEC50: 0.140nMAssay Description:The compound of Example 3 was tested for agonist activity at the rat CB1 (rCB1) and rCB2 receptors, at eight concentrations, in duplicate: 10, 3, 1, ...More data for this Ligand-Target Pair
Affinity DataEC50: 21.7nMAssay Description:The compound of Example 3 was tested for agonist activity at the rat CB1 (rCB1) and rCB2 receptors, at eight concentrations, in duplicate: 10, 3, 1, ...More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Functional activity was evaluated using GTPγ[35S] assay in CHO membrane extracts expressing recombinant hCB1 (human CB1) receptors or hCB2 (huma...More data for this Ligand-Target Pair
Affinity DataEC50: 409nMAssay Description:Functional activity was evaluated using GTPγ[35S] assay in CHO membrane extracts expressing recombinant hCB1 (human CB1) receptors or hCB2 (huma...More data for this Ligand-Target Pair
Affinity DataEC50: 1.13nMAssay Description:The compound of Example 3 was tested for agonist activity at the rat CB1 (rCB1) and rCB2 receptors, at eight concentrations, in duplicate: 10, 3, 1, ...More data for this Ligand-Target Pair
Affinity DataEC50: 479nMAssay Description:Functional activity was evaluated using GTPγ[35S] assay in CHO membrane extracts expressing recombinant hCB1 (human CB1) receptors or hCB2 (huma...More data for this Ligand-Target Pair
Affinity DataEC50: 160nMAssay Description:Functional activity was evaluated using GTPγ[35S] assay in CHO membrane extracts expressing recombinant hCB1 (human CB1) receptors or hCB2 (huma...More data for this Ligand-Target Pair

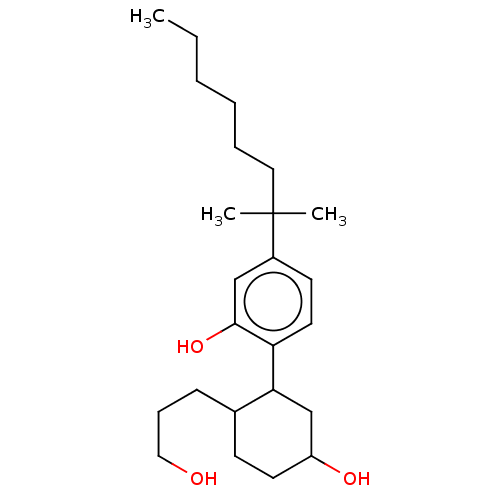
 3D Structure (crystal)
3D Structure (crystal)