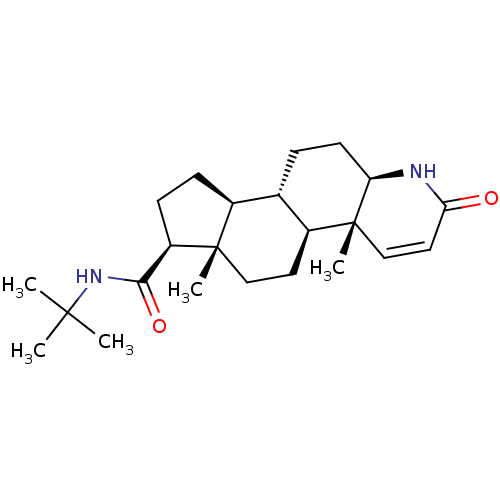
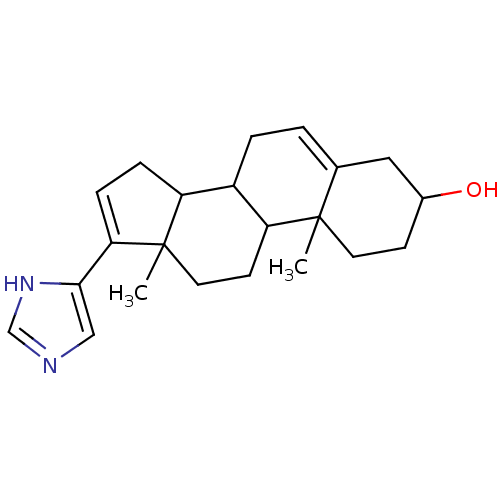
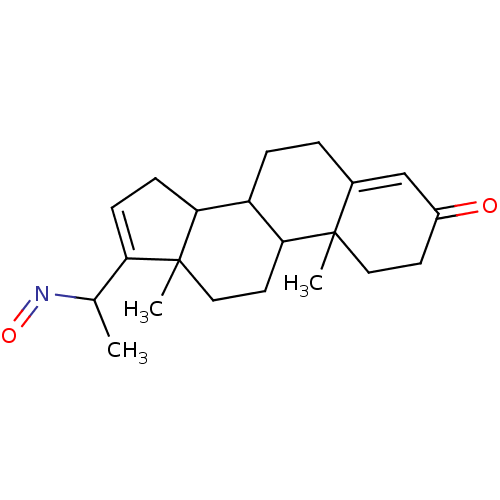
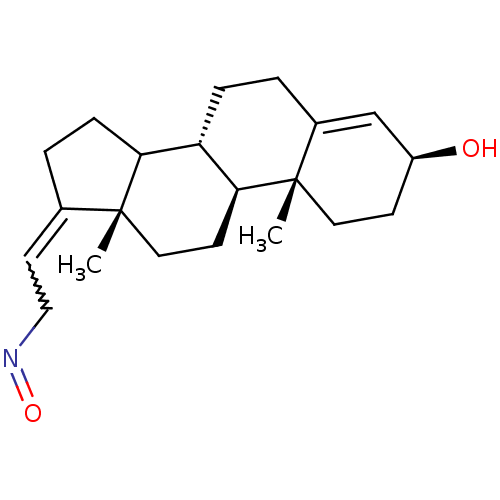
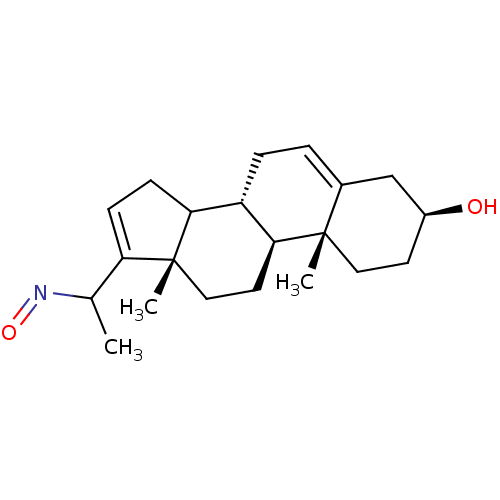
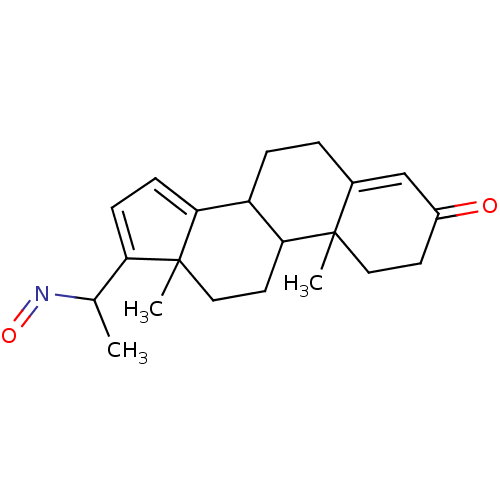
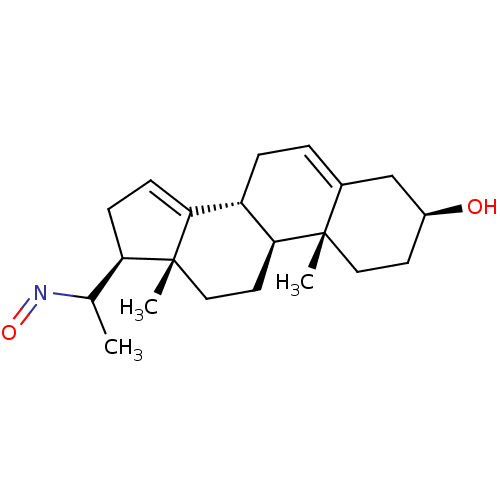
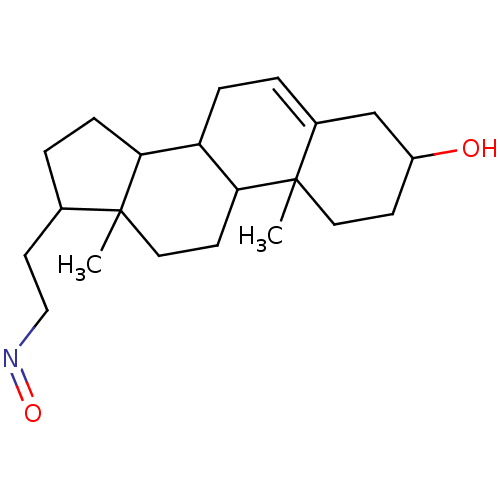
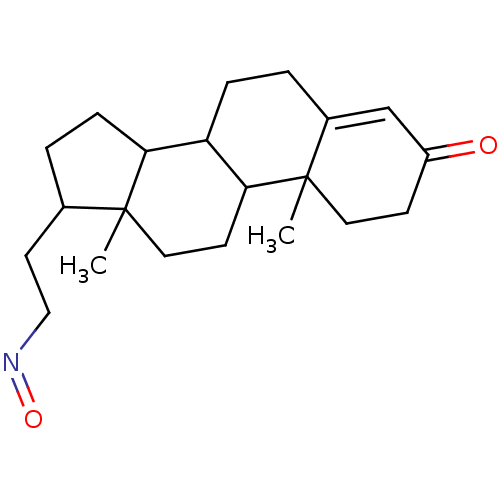
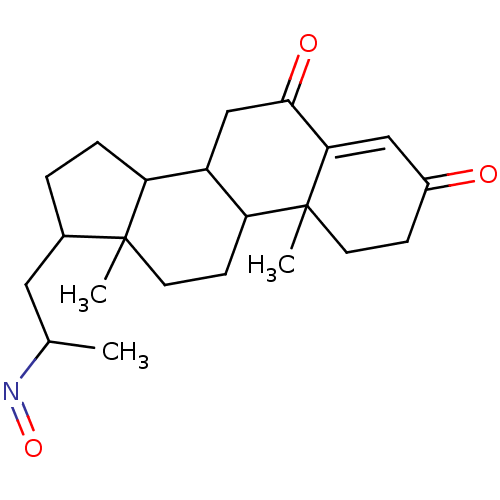
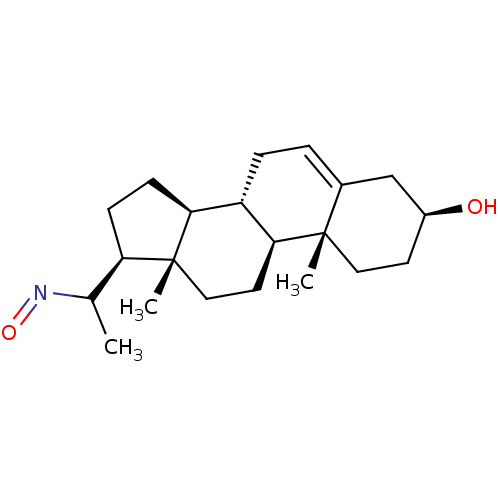
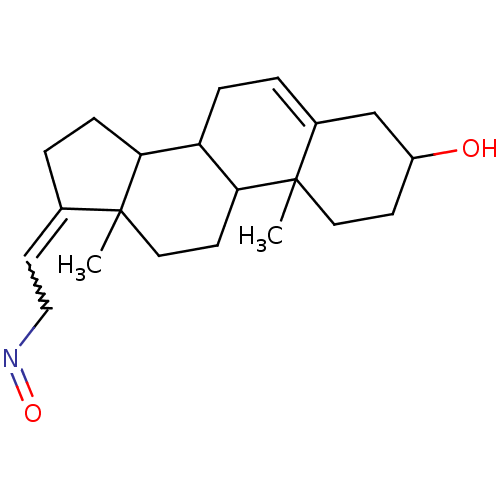
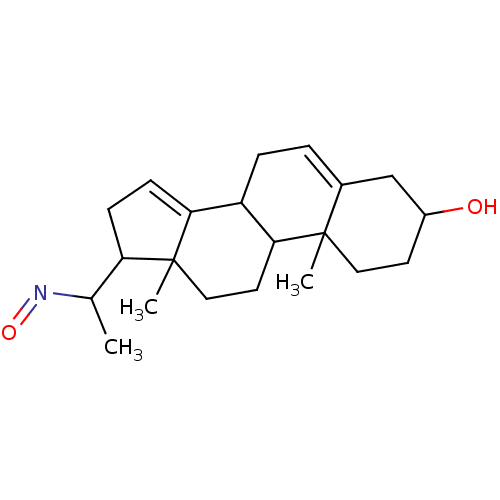
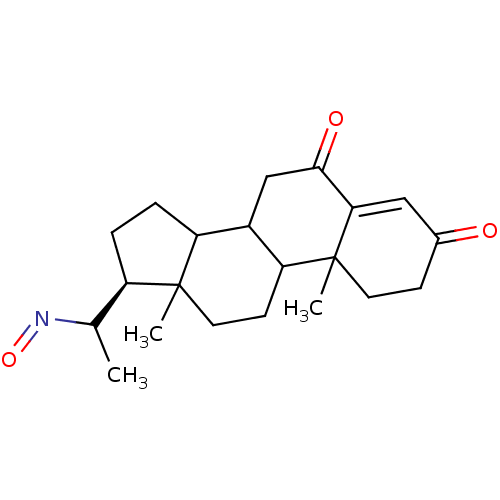
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human Cytochrome P450 17More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type II expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 77nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 170nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of rat Cytochrome P450 17More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of Human P450 17 and NADPH-P450 reductase co-expressed in Escherichia coli with 25 uM progesteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 420nMAssay Description:Inhibition of Human P450 17 and NADPH-P450 reductase co-expressed in Escherichia coli with 25 uM progesteroneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 430nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 430nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 470nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 520nMAssay Description:Inhibition of Human P450 17 and NADPH-P450 reductase co-expressed in Escherichia coli with 25 uM progesteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 520nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 520nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type I expressed in HEK293 cellsMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 580nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 580nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 740nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 860nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type II expressed in HEK293 cellsMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 890nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type II expressed in HEK293 cellsMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 1.18E+3nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 1.63E+3nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 1.65E+3nMAssay Description:Inhibition of Cytochrome P450 17 of human testicular microsomes at 25 uM progesteroneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 1.77E+3nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type I expressed in HEK293 cellsMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 1.95E+3nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 2.44E+3nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type I expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: >2.50E+3nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: >2.50E+3nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: >2.50E+3nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: >2.50E+3nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: >2.50E+3nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 human enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 2.76E+3nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of Human P450 17 and NADPH-P450 reductase co-expressed in Escherichia coli with 25 uM progesteroneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of Human steroid 5-alpha-reductase type I expressed in HEK293 cellsMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 3.20E+3nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type 2 from human BPH tissue at 210 nM of testosteroneMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 3.60E+3nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: 9.80E+3nMAssay Description:Evaluated for the inhibitory activity towards Cytochrome P450 17 rat enzyme using testicular microsome at 25 uM of substrate (progesterone)More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Of The Saarland
Curated by ChEMBL
University Of The Saarland
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Evaluated for the inhibitory activity against human steroid 5-alpha-reductase type I in human DU-145 cell assay at 5 nM of androstenedioneMore data for this Ligand-Target Pair