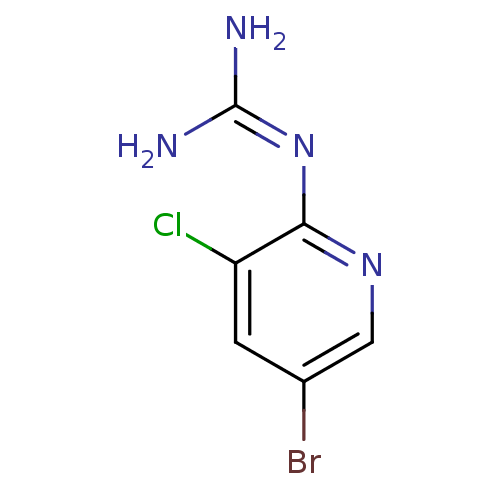
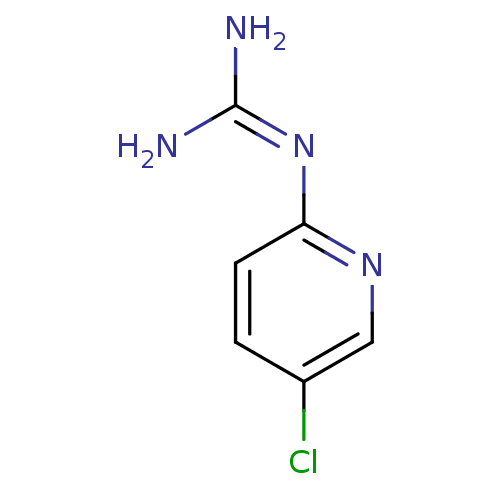
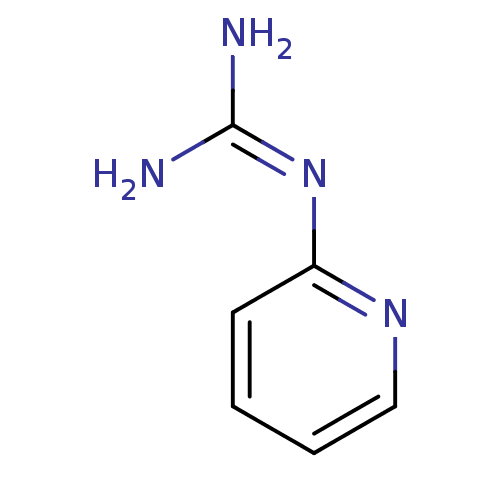
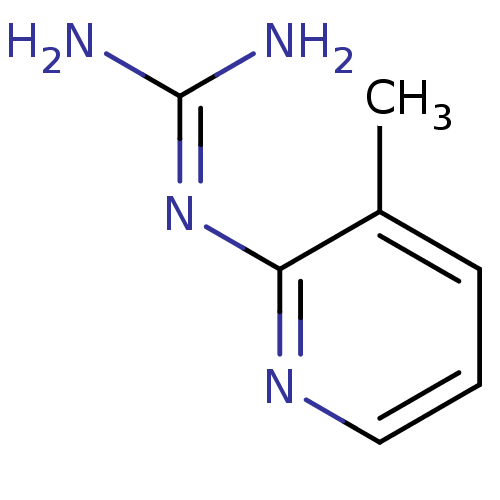
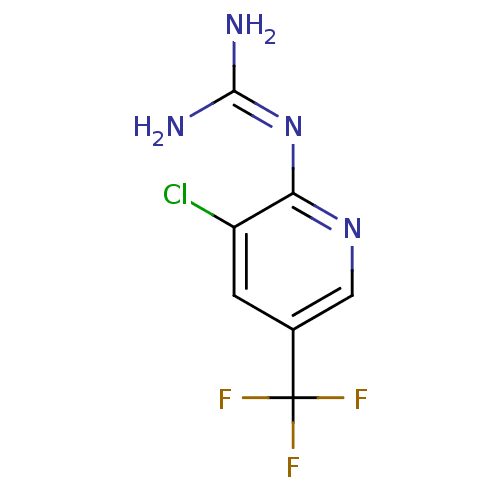
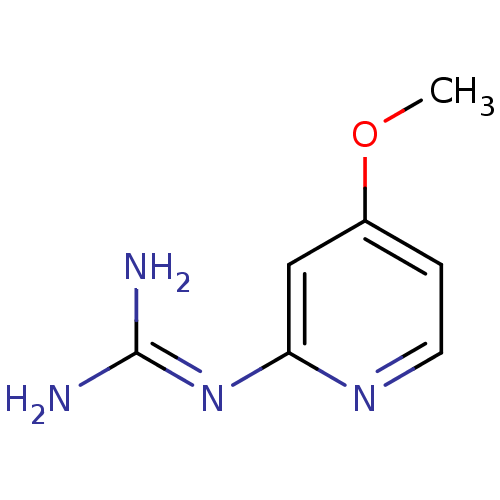
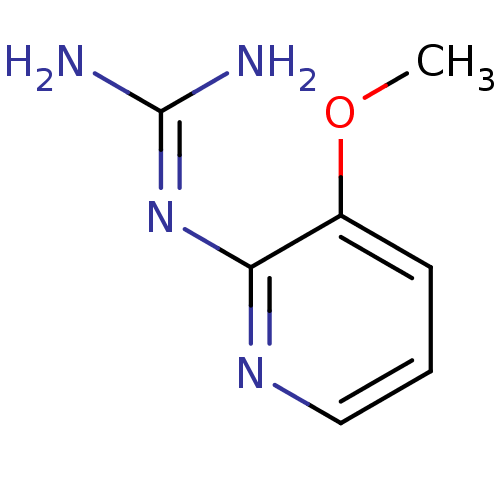
Affinity DataKi: 2.90E+3nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
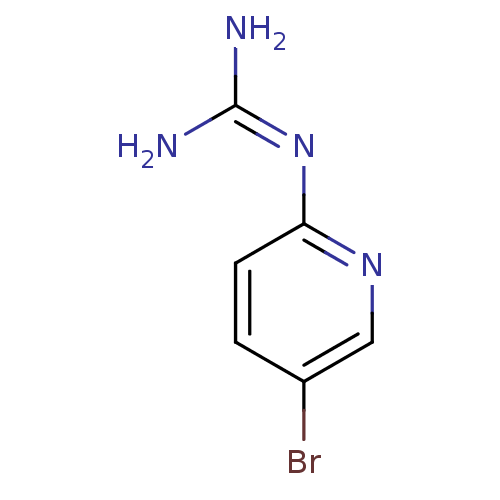
Affinity DataKi: 3.13E+3nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
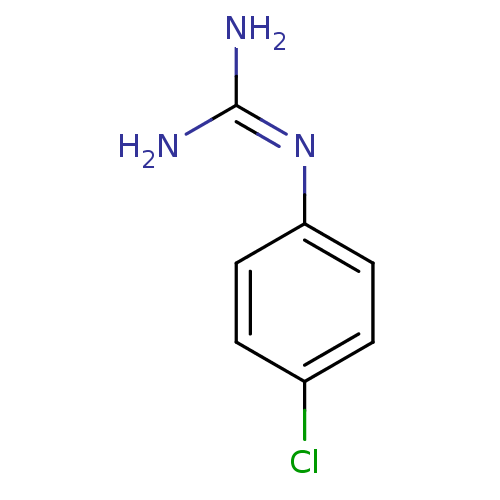
Affinity DataKi: 4.83E+3nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
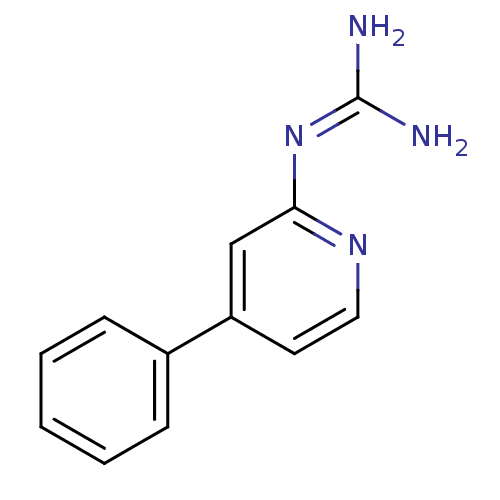
Affinity DataKi: 5.47E+3nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 6.70E+3nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 7.10E+3nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 8.70E+3nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 1.69E+4nMAssay Description:Ability to inhibit human plasmin using Chromozym-PL as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 2.95E+4nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 3.23E+4nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 3.77E+4nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 4.07E+4nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: <5.00E+4nMAssay Description:Ability to inhibit human plasmin using Chromozym-PL as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 5.33E+4nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 6.20E+4nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 8.38E+4nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 1.47E+5nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 1.73E+5nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair
Affinity DataKi: 1.77E+5nMAssay Description:Ability to inhibit human plasmin using Chromozym-PL as substrateMore data for this Ligand-Target Pair
Affinity DataKi: <2.33E+5nMAssay Description:Ability to inhibit human plasmin using Chromozym-PL as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 2.45E+5nMAssay Description:Ability to inhibit human tissue plasminogen activator stimulatorMore data for this Ligand-Target Pair
Affinity DataKi: 2.72E+5nMAssay Description:Ability to inhibit human tissue plasminogen activator stimulatorMore data for this Ligand-Target Pair
Affinity DataKi: 2.92E+5nMAssay Description:In vitro inhibition of HWMT human urokinase Plasminogen activator.More data for this Ligand-Target Pair