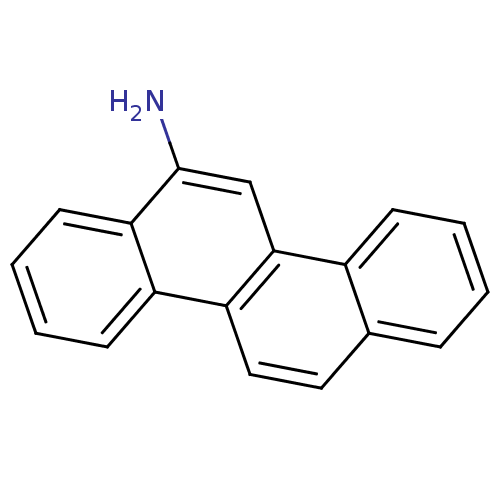
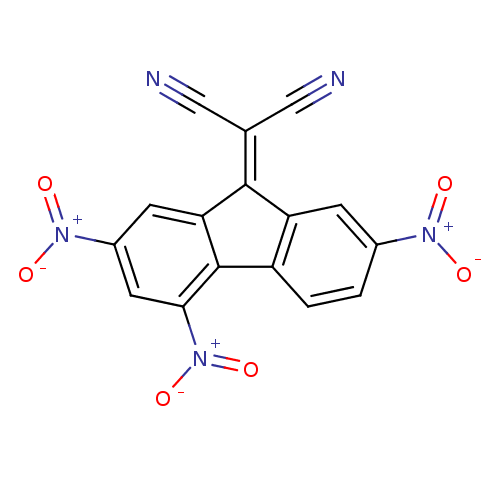
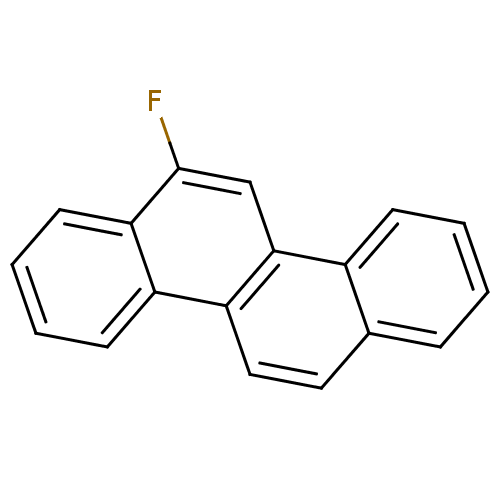
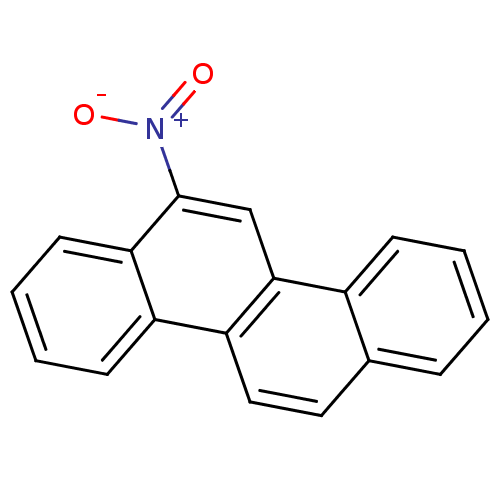
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 500nMAssay Description:Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 600nMAssay Description:Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 2.20E+3nMAssay Description:Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 2.80E+3nMAssay Description:Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 5.40E+3nMAssay Description:Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 6.70E+3nMAssay Description:Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 8.98E+3nMAssay Description:Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 1.09E+4nMAssay Description:Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 1.18E+4nMAssay Description:Kinetic inhibition constant of compound with Trypanosoma cruzi Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 1.39E+4nMAssay Description:Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: >2.50E+4nMAssay Description:Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 2.88E+4nMAssay Description:Kinetic inhibition constant of compound with human Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair
TargetHypoxanthine-guanine phosphoribosyltransferase(Homo sapiens (Human))
University Of Connecticut
Curated by ChEMBL
University Of Connecticut
Curated by ChEMBL
Affinity DataKi: 4.81E+4nMAssay Description:Kinetic inhibition constant of compound with bacterial Hypoxanthine Phosphoribosyltransferase (HPRT)More data for this Ligand-Target Pair