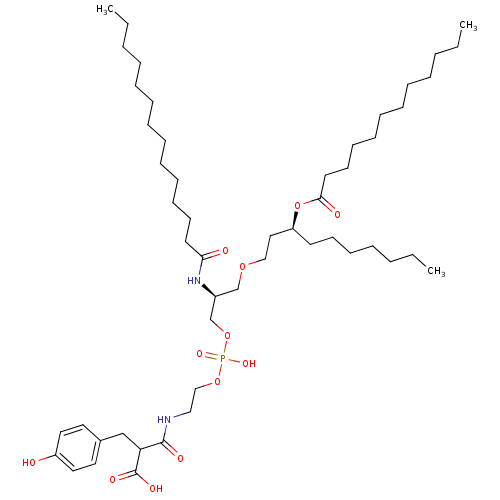
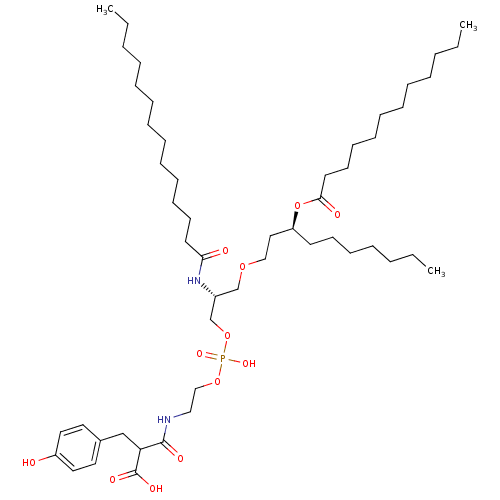
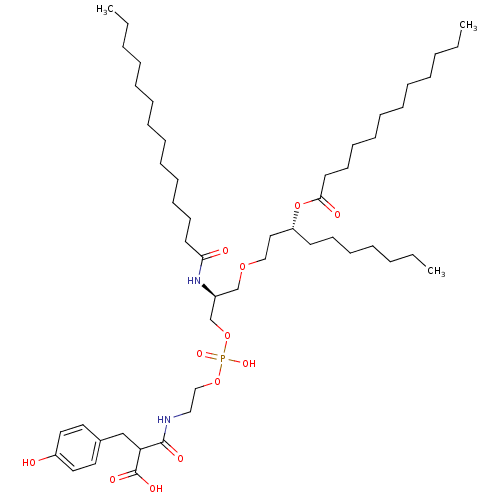
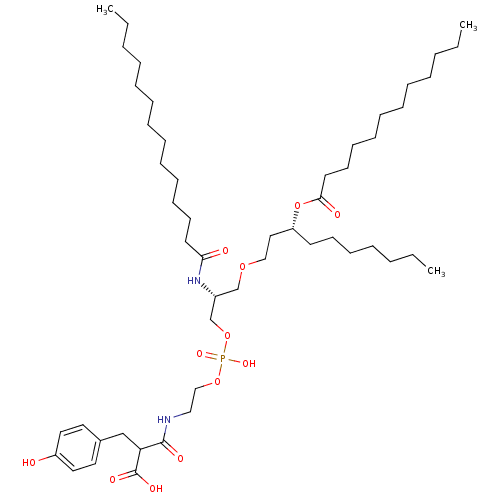
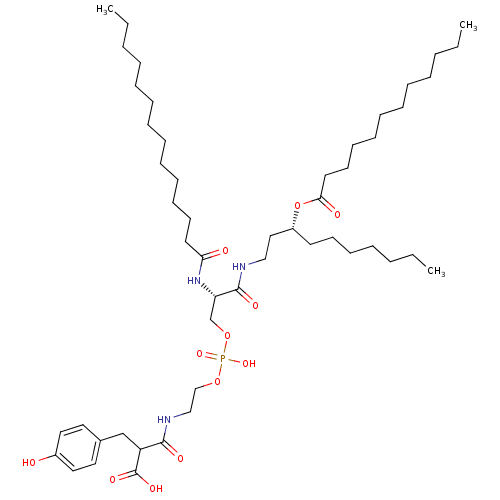
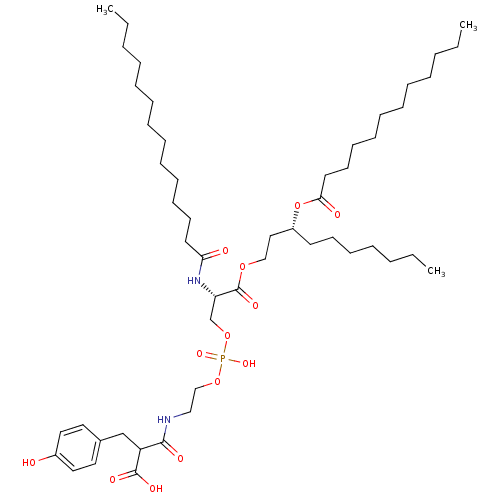
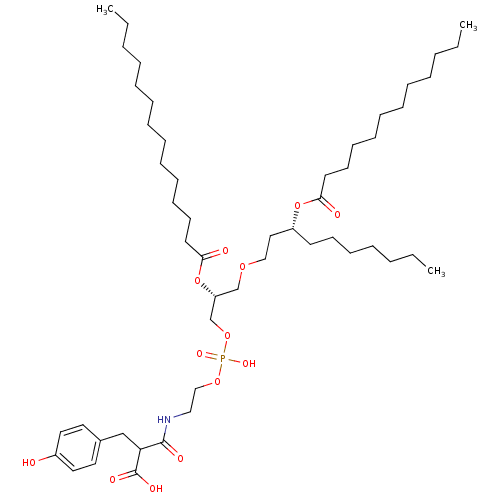
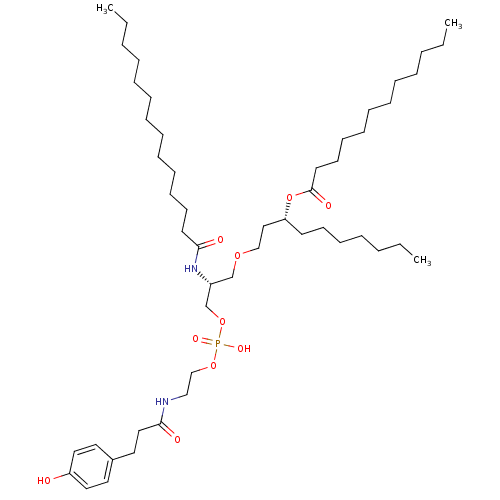
Affinity DataIC50: 80nMAssay Description:Antagonistic potency at human TLR4 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Antagonistic potency at human TLR4 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 250nMAssay Description:Antagonistic potency at human TLR4 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Antagonistic potency at human TLR4 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Antagonistic potency at TLR2 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 950nMAssay Description:Antagonistic potency at TLR2 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+3nMAssay Description:Antagonistic potency at TLR2 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Antagonistic activity at TLR in human PBMCMore data for this Ligand-Target Pair
Affinity DataIC50: 2.15E+3nMAssay Description:Antagonistic potency at TLR2 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.07E+3nMAssay Description:Antagonistic potency at TLR2 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+3nMAssay Description:Antagonistic potency at TLR2 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.46E+3nMAssay Description:Antagonistic potency at TLR2 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90E+3nMAssay Description:Antagonistic potency at TLR2 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Antagonistic potency at TLR2 expressed in HEK293 cellsMore data for this Ligand-Target Pair