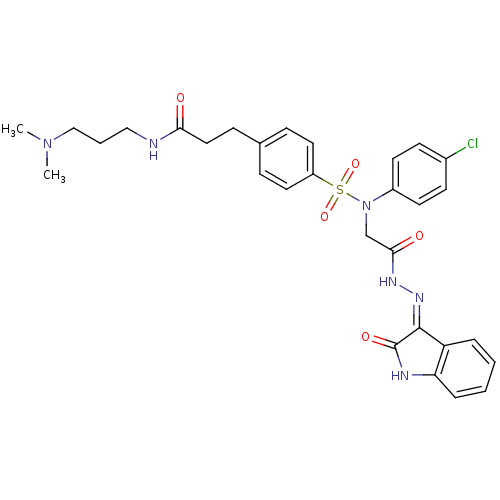
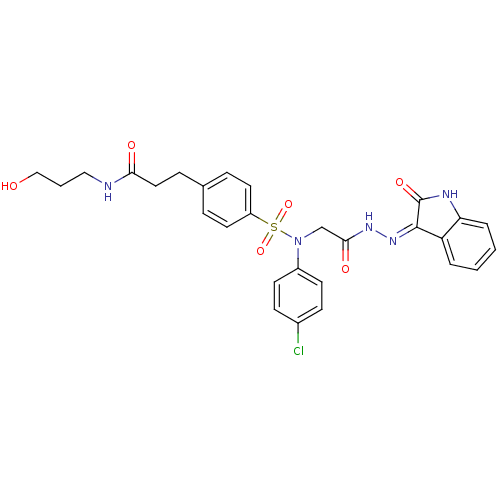
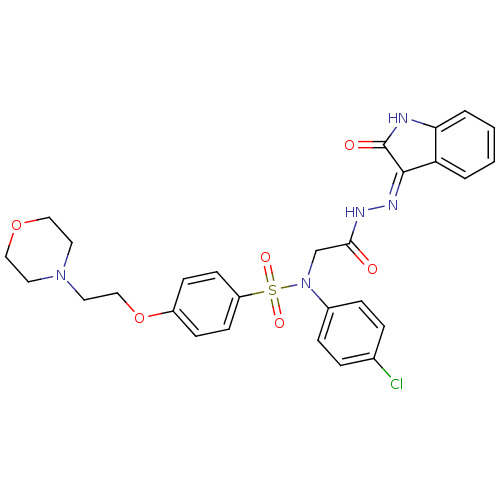
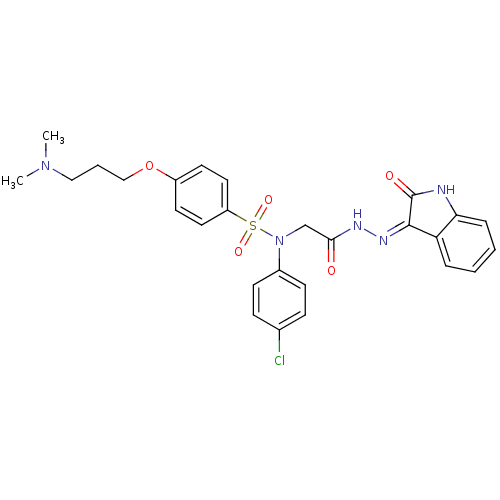
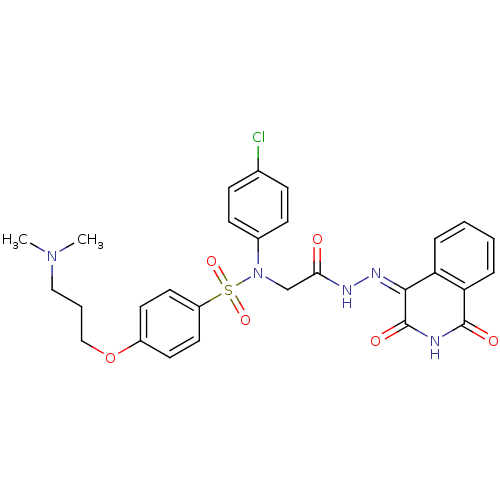
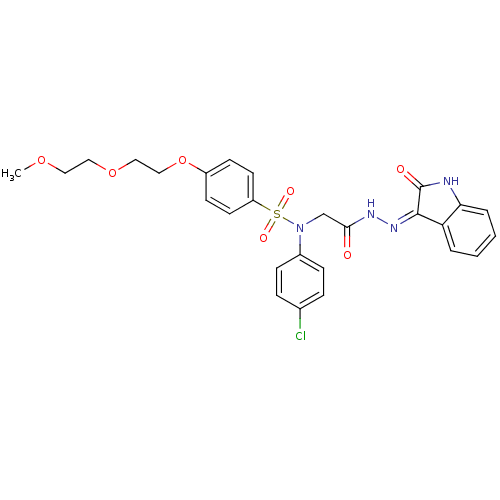
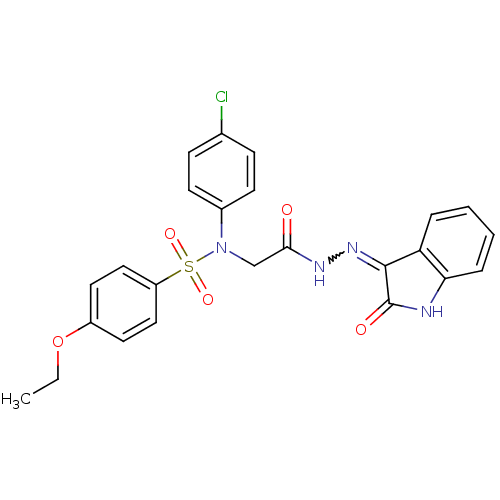
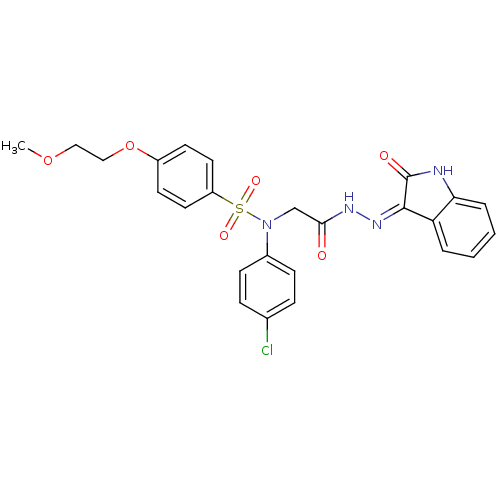
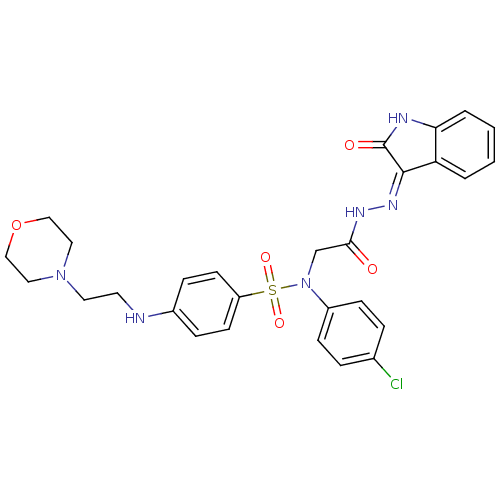
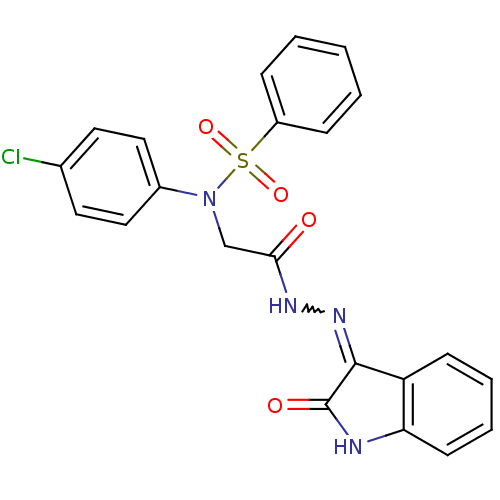
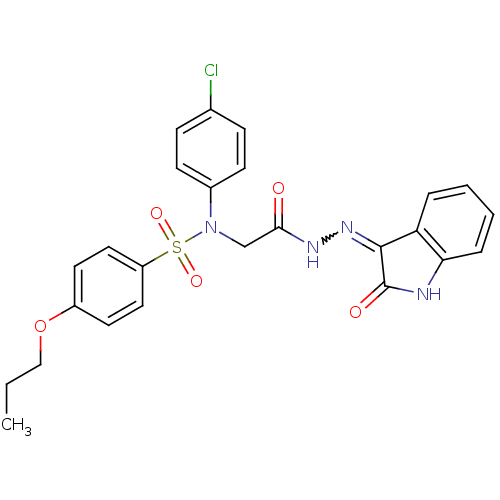
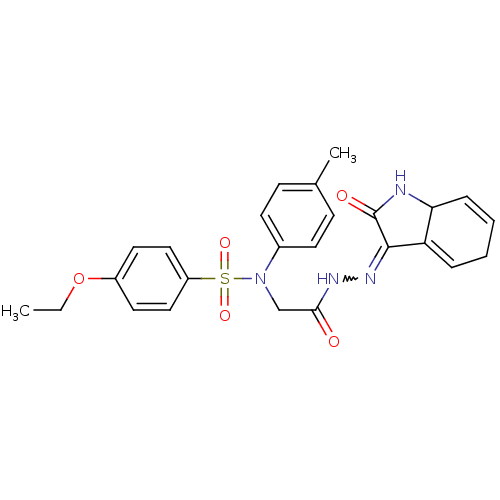
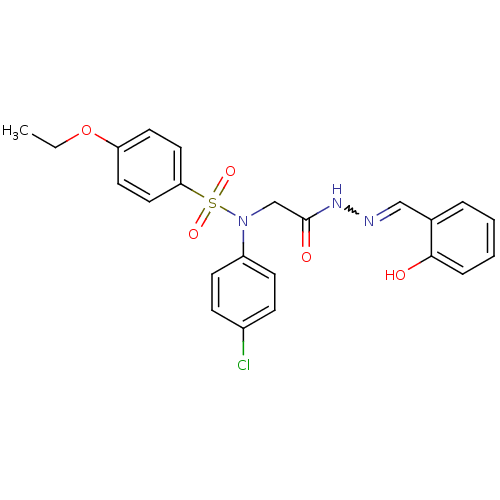
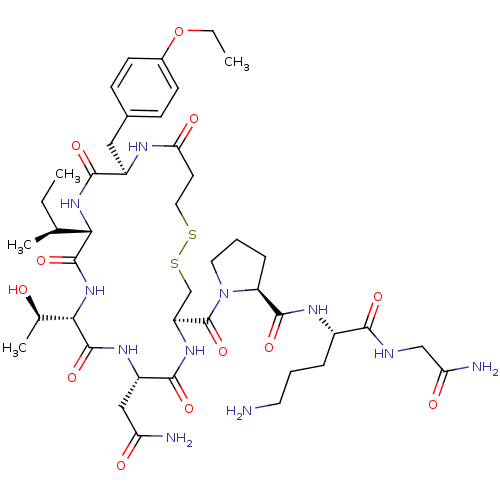
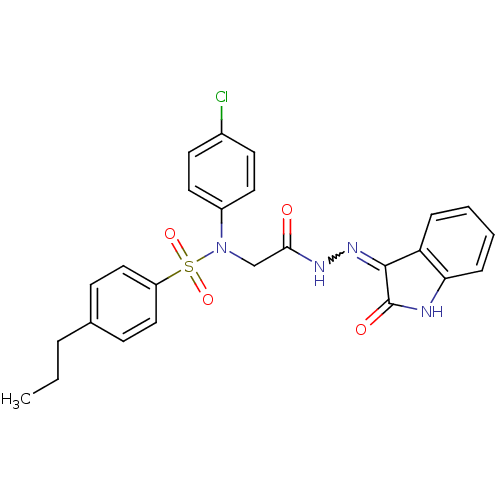
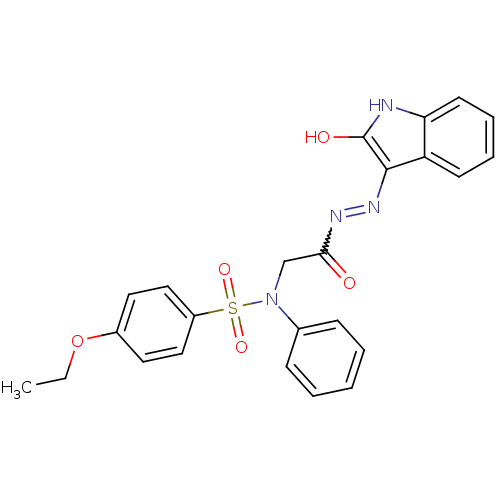
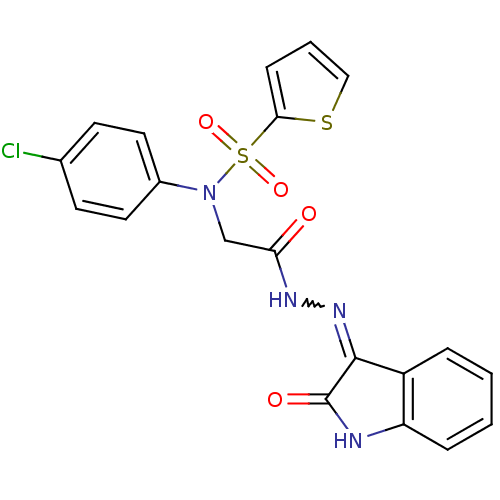
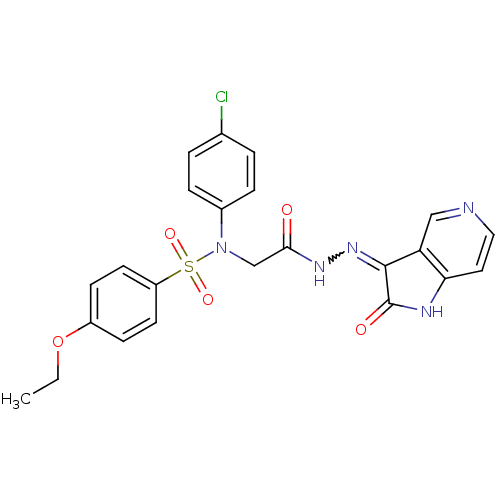
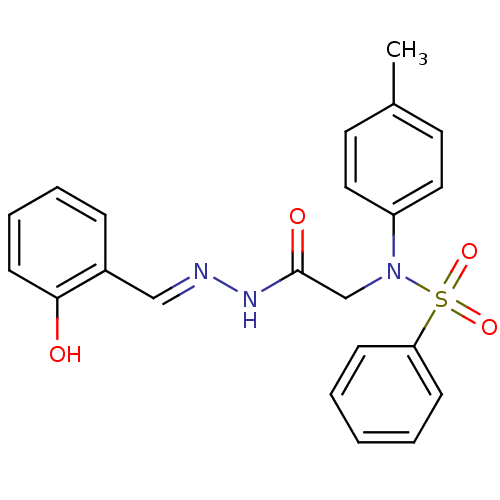
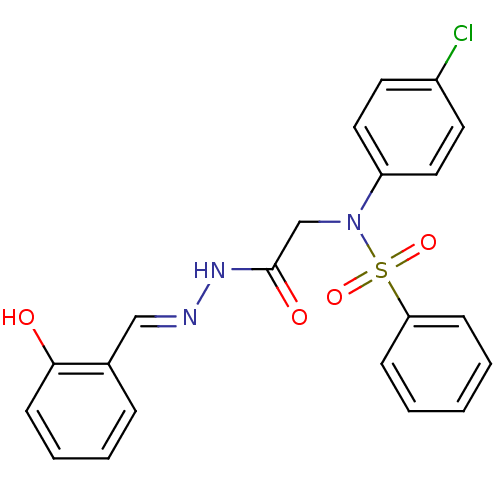
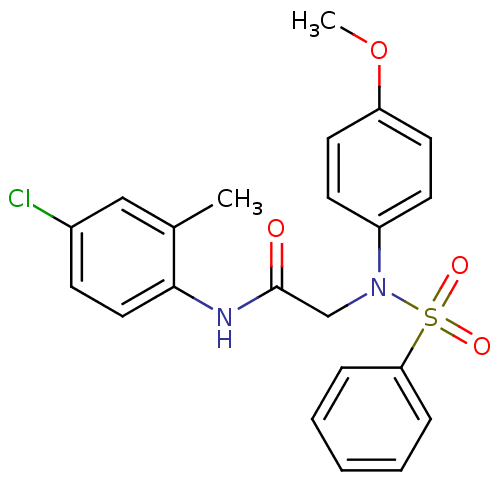
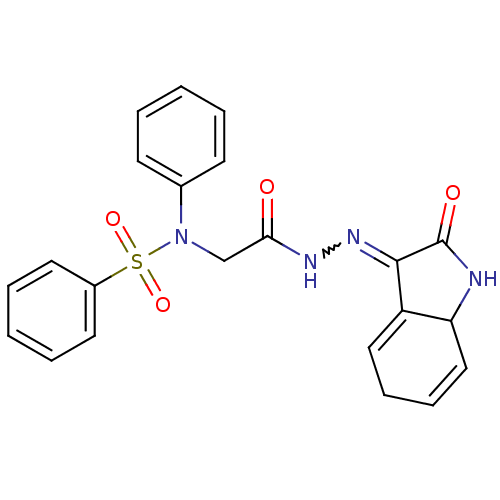
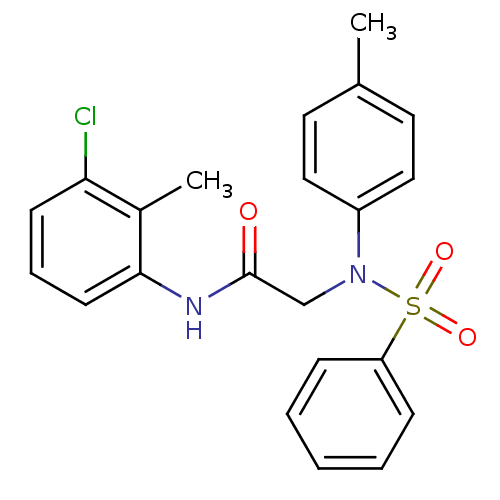
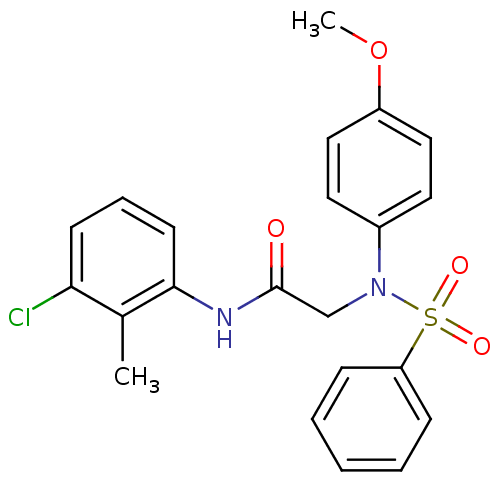
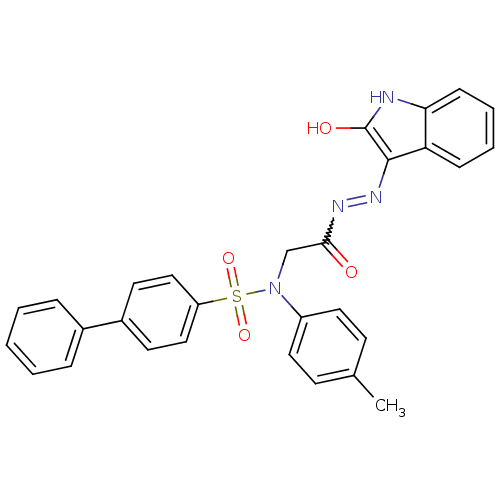
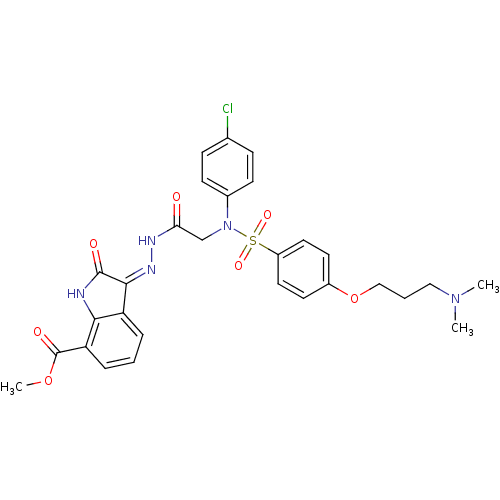
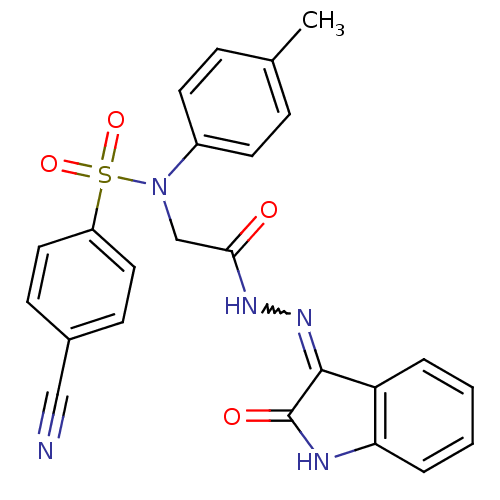
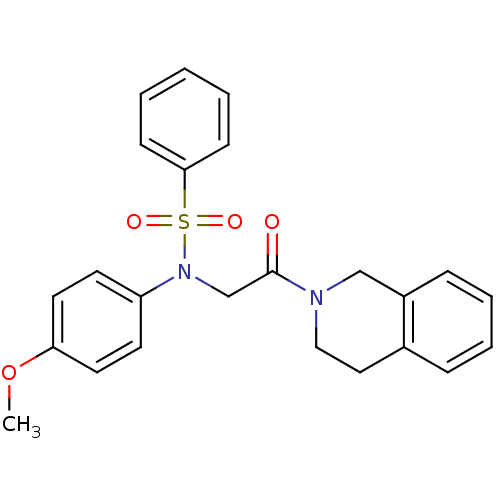
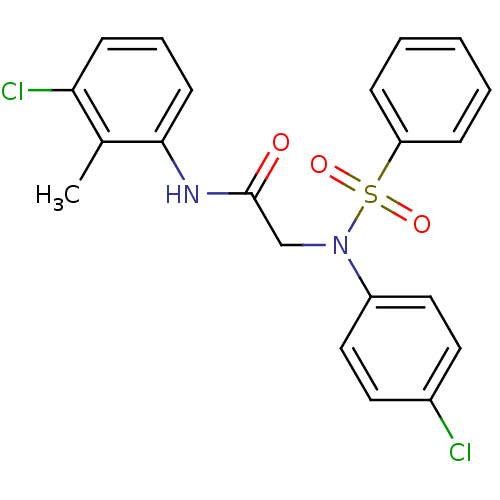
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.650nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.670nMAssay Description:Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
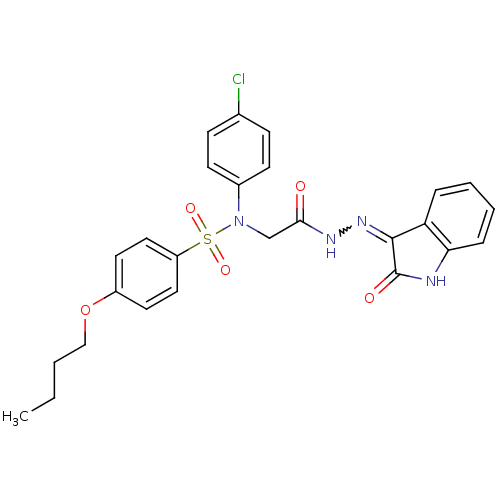
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
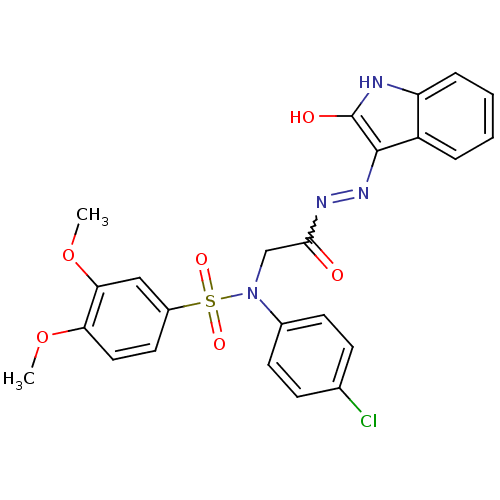
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
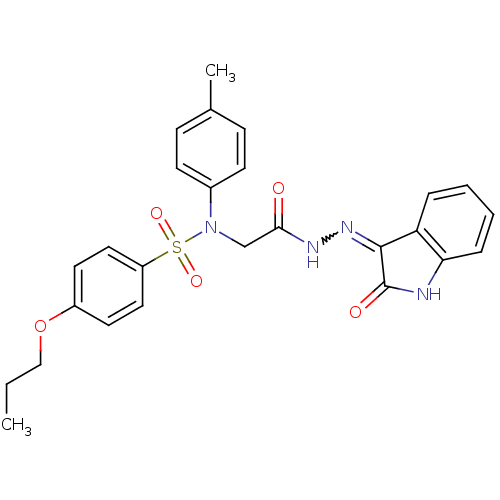
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.10nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.90nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 4.10nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.60nMAssay Description:Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 8.70nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 21nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 36nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 36nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 37nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 61nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 64nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 67nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 67nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetVasopressin V1a receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 70nMAssay Description:Displacement of [125I]LVA antagonist from human vasopressin 1a receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
Affinity DataKi: 76nMAssay Description:Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 93nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 100nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 104nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 109nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 111nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 115nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetVasopressin V1a receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 115nMAssay Description:Displacement of [125I]LVA antagonist from human vasopressin 1a receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Displacement of [125I]OVTA antagonist from rat oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 160nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Serono Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 160nMAssay Description:Displacement of [125I]OVTA antagonist from human oxytocin receptor expressed in HEK293-EBNA cellsMore data for this Ligand-Target Pair