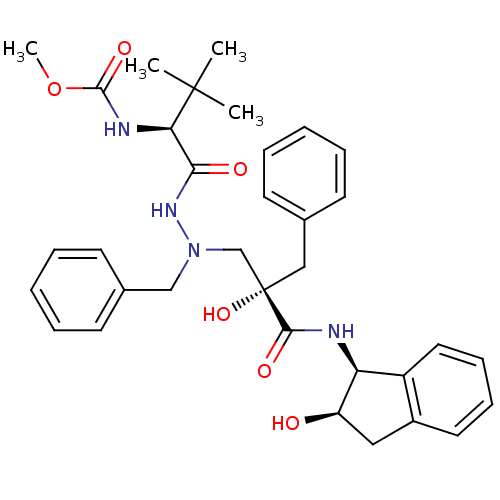
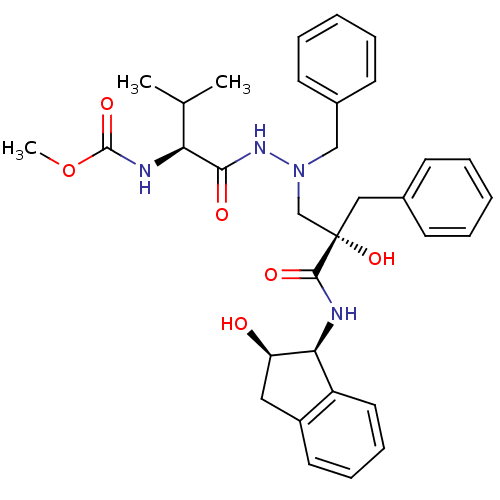
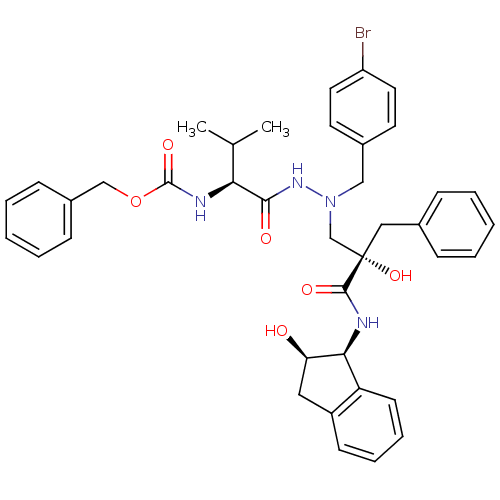
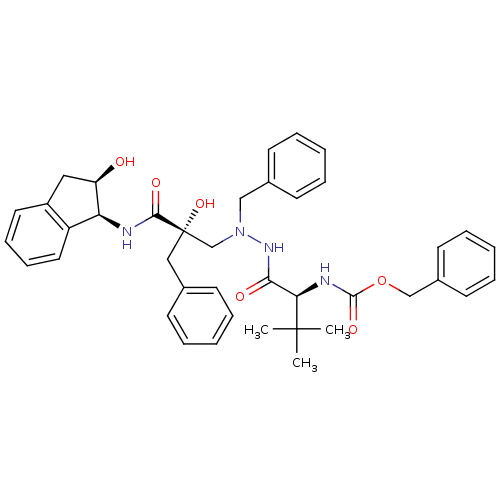
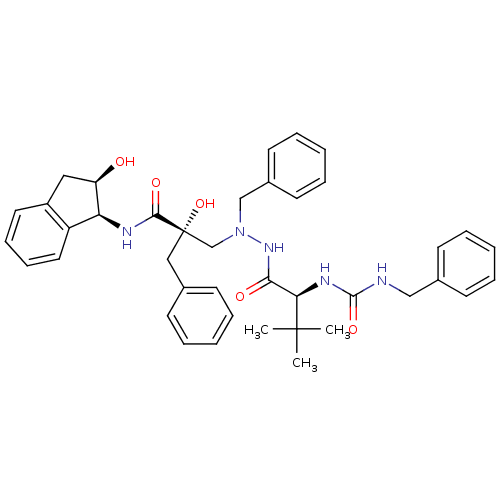
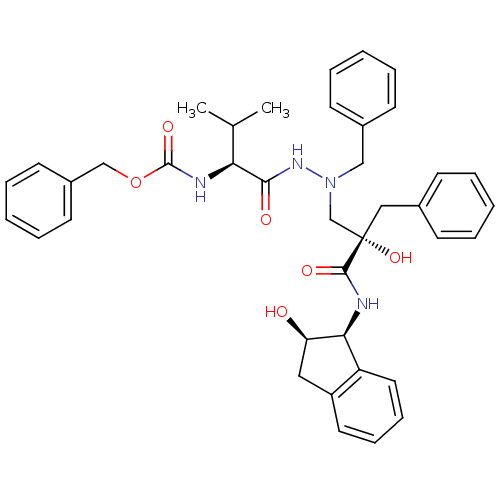
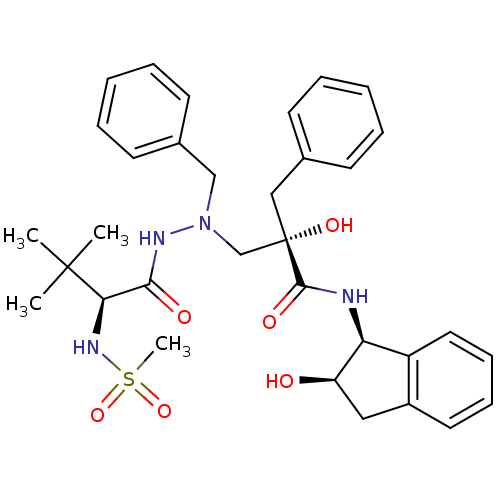
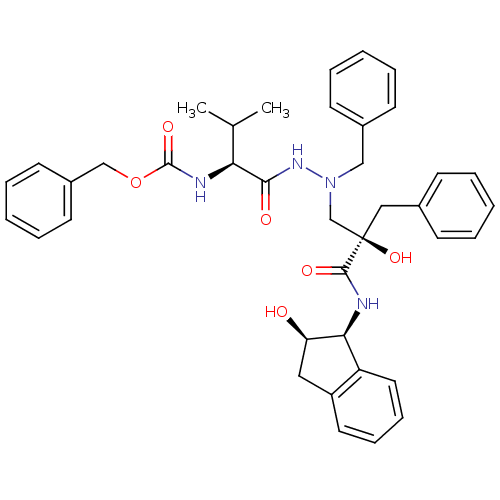
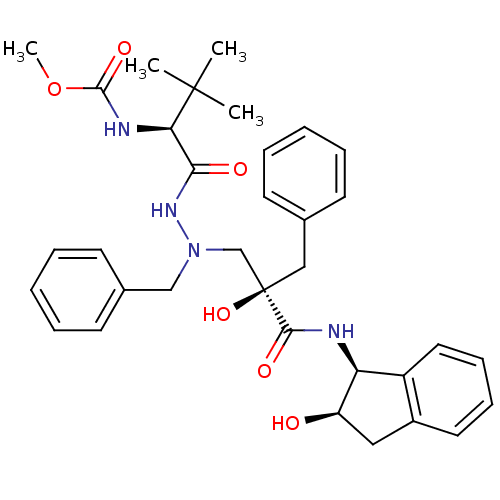
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Uppsala University
Curated by ChEMBL
Uppsala University
Curated by ChEMBL
Affinity DataKi: 2.40nMAssay Description:Inhibitory activity against HIV1 protease expressed in Escherichia coli in a fluorometric assayMore data for this Ligand-Target Pair
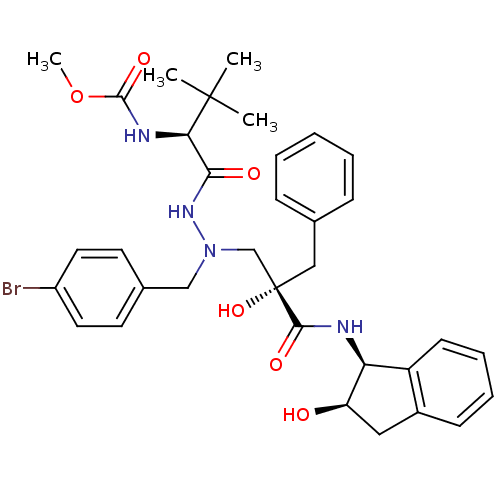
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Uppsala University
Curated by ChEMBL
Uppsala University
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Inhibitory activity against HIV1 protease expressed in Escherichia coli in a fluorometric assayMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Uppsala University
Curated by ChEMBL
Uppsala University
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibitory activity against HIV1 protease expressed in Escherichia coli in a fluorometric assayMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Uppsala University
Curated by ChEMBL
Uppsala University
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Inhibitory activity against HIV1 protease expressed in Escherichia coli in a fluorometric assayMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Uppsala University
Curated by ChEMBL
Uppsala University
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Inhibitory activity against HIV1 protease expressed in Escherichia coli in a fluorometric assayMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Uppsala University
Curated by ChEMBL
Uppsala University
Curated by ChEMBL
Affinity DataKi: 22nMAssay Description:Inhibitory activity against HIV1 protease expressed in Escherichia coli in a fluorometric assayMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Uppsala University
Curated by ChEMBL
Uppsala University
Curated by ChEMBL
Affinity DataKi: 23nMAssay Description:Inhibitory activity against HIV1 protease expressed in Escherichia coli in a fluorometric assayMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Uppsala University
Curated by ChEMBL
Uppsala University
Curated by ChEMBL
Affinity DataKi: 63nMAssay Description:Inhibitory activity against HIV1 protease expressed in Escherichia coli in a fluorometric assayMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Uppsala University
Curated by ChEMBL
Uppsala University
Curated by ChEMBL
Affinity DataKi: 870nMAssay Description:Inhibitory activity against HIV1 protease expressed in Escherichia coli in a fluorometric assayMore data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Uppsala University
Curated by ChEMBL
Uppsala University
Curated by ChEMBL
Affinity DataKi: 2.20E+3nMAssay Description:Inhibitory activity against HIV1 protease expressed in Escherichia coli in a fluorometric assayMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)