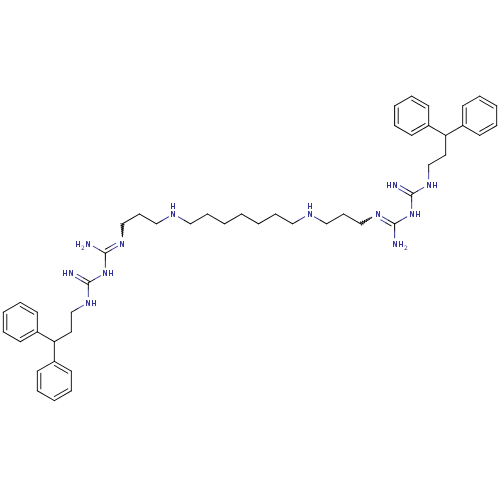
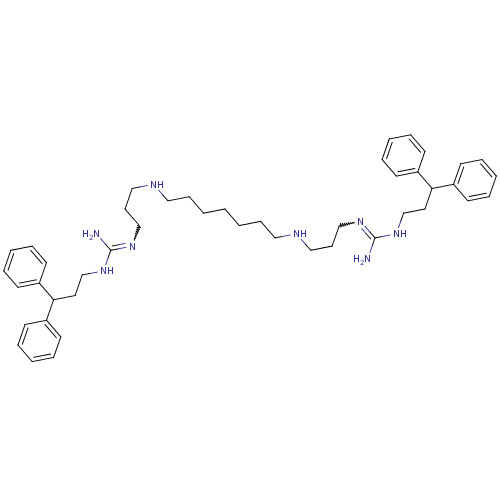
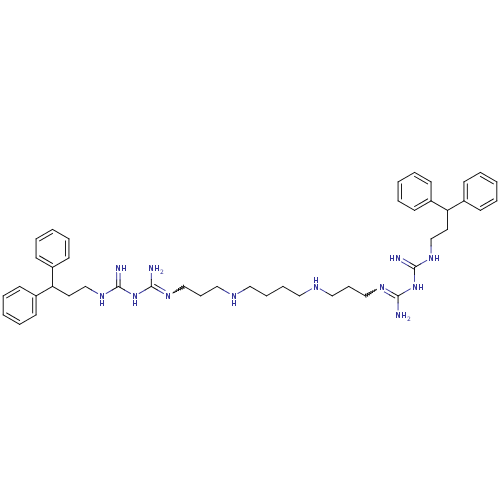
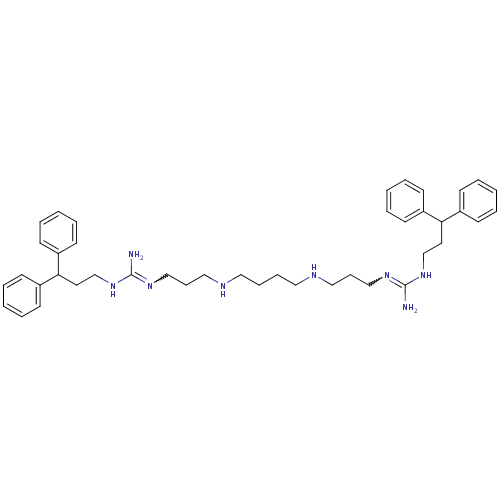
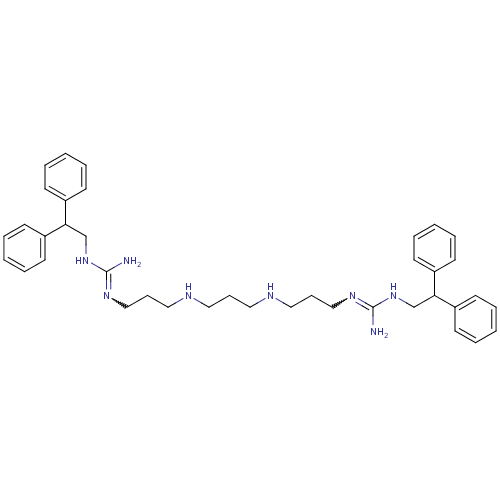
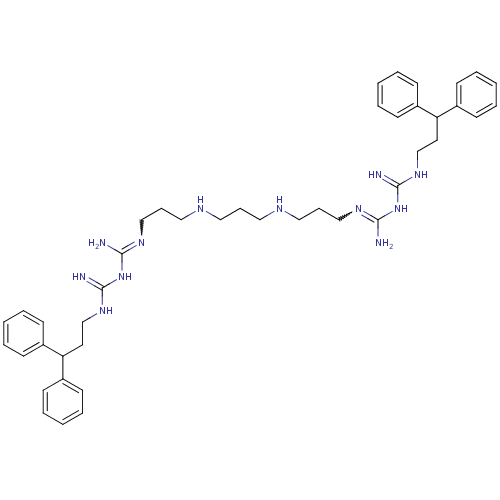
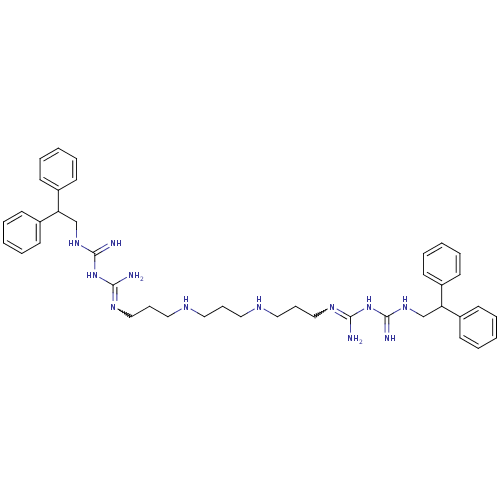
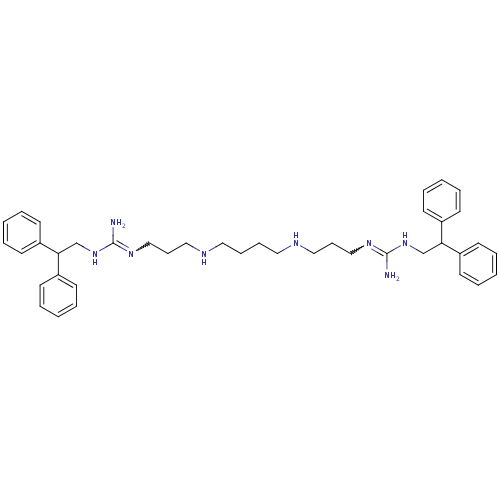
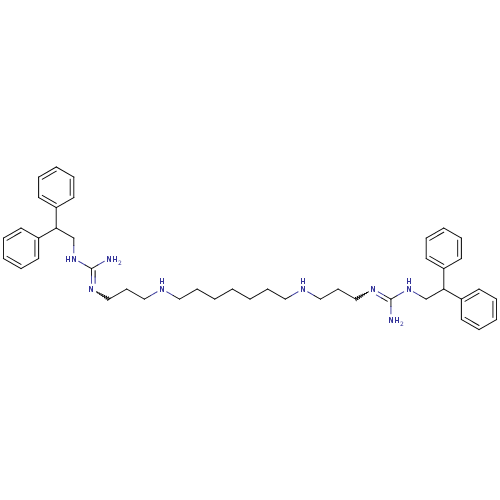
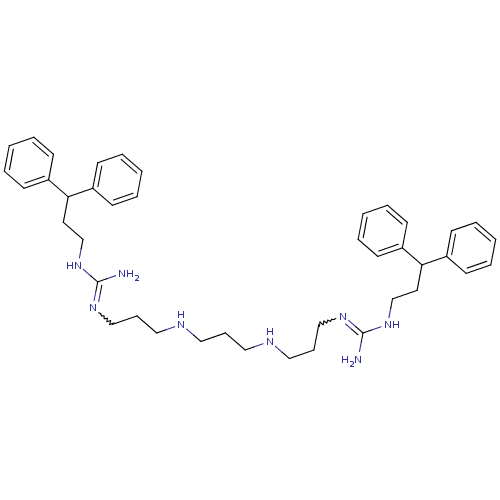
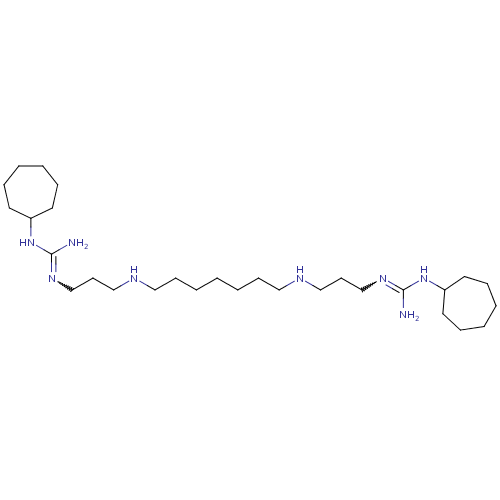
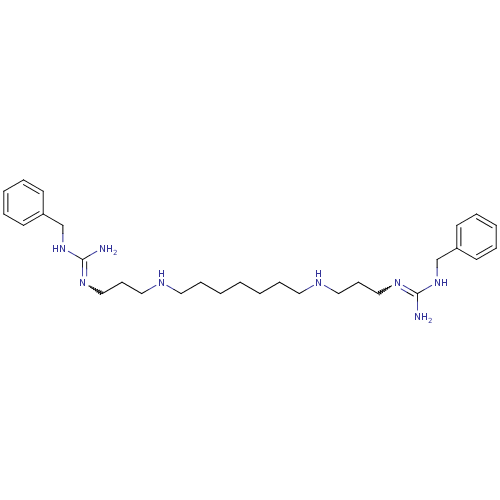
Affinity DataIC50: 950nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 1.66E+3nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 2.24E+3nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 2.74E+3nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 2.96E+3nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 2.97E+3nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 3.68E+3nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 4.16E+3nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 4.56E+3nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 4.72E+3nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 5.07E+3nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 1.77E+4nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
Affinity DataIC50: 6.95E+4nMAssay Description:Inhibition of Trypanosoma cruzi TRMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human glutathione reductaseMore data for this Ligand-Target Pair