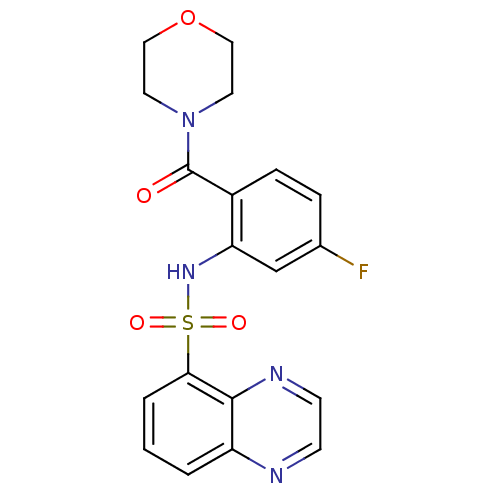
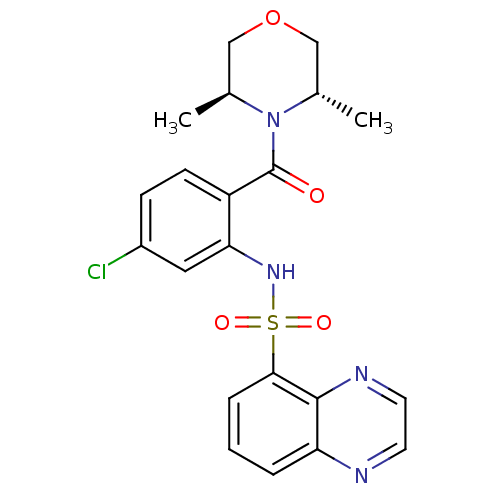
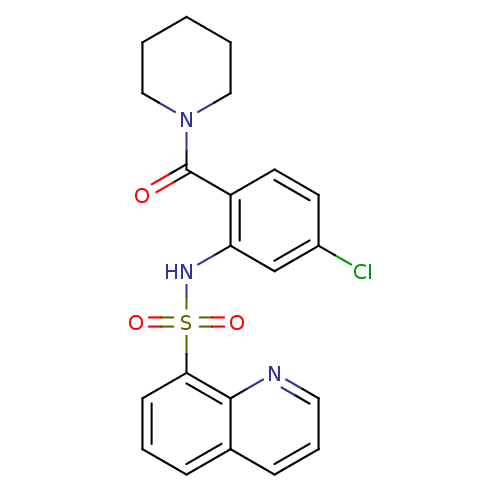
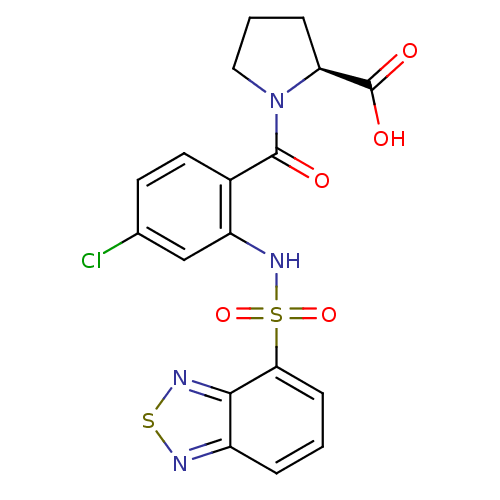
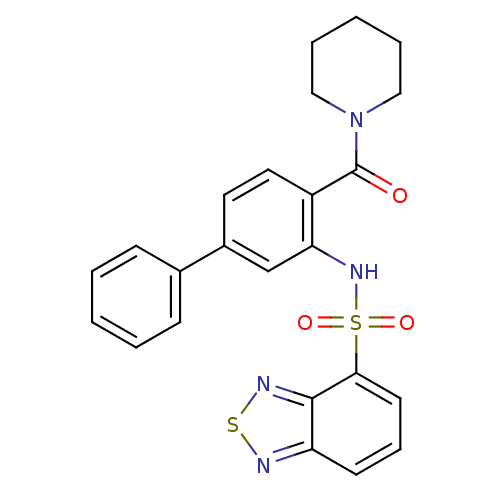
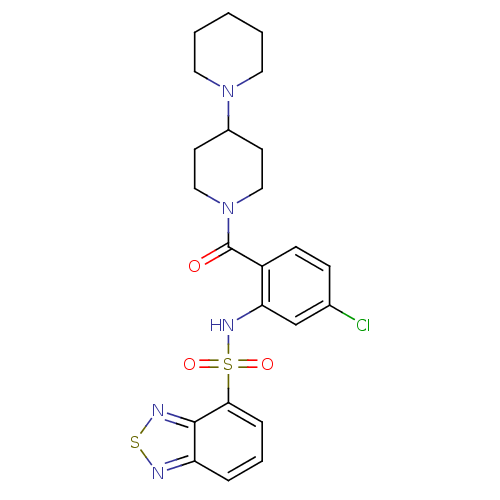
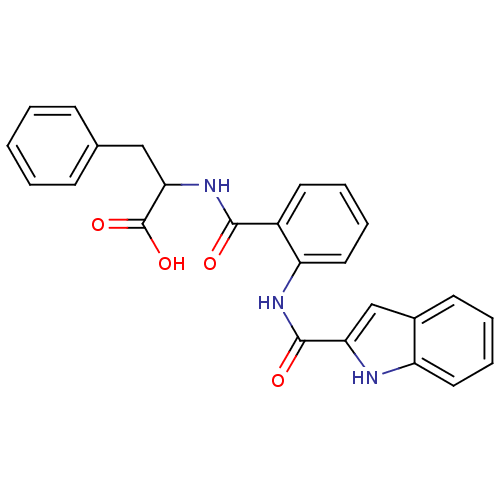
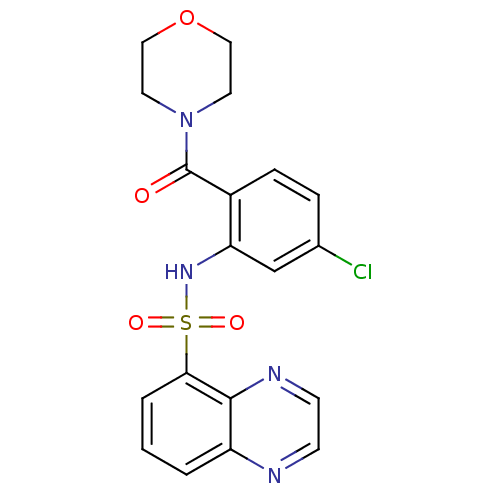
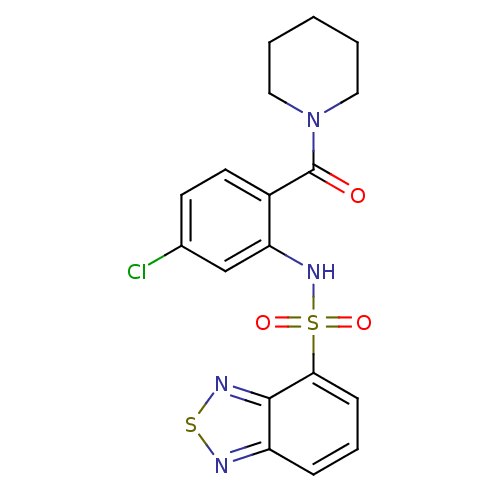
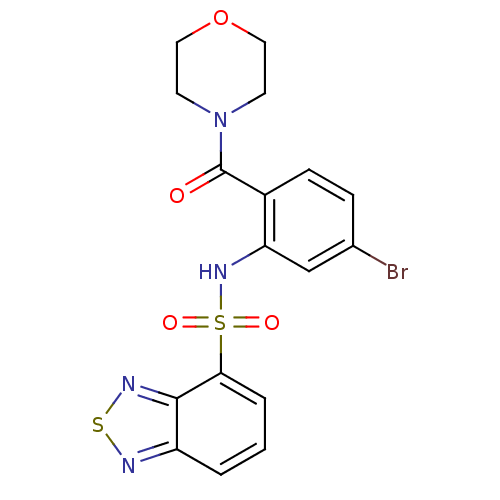
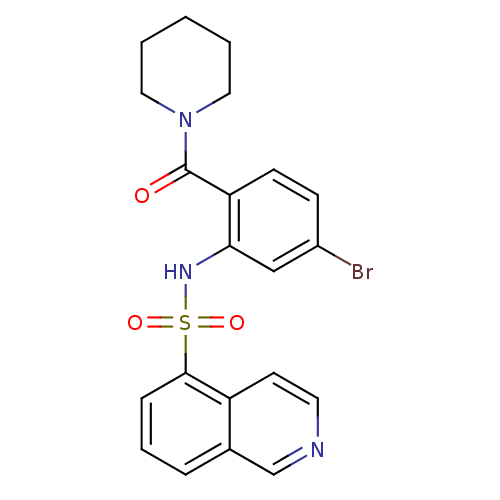
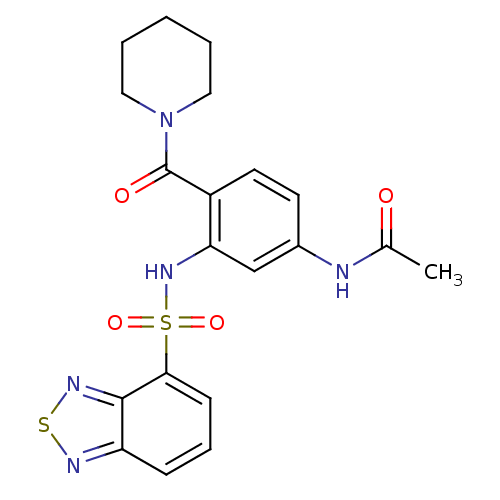
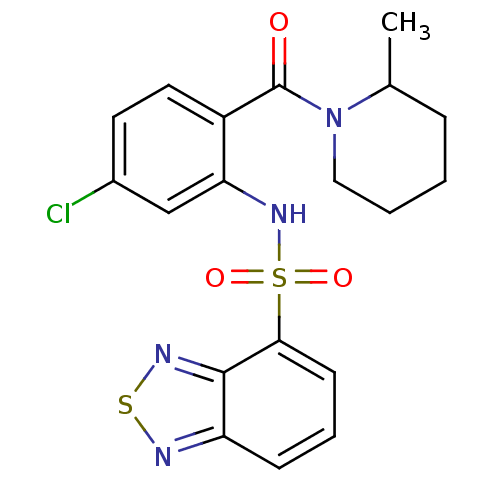
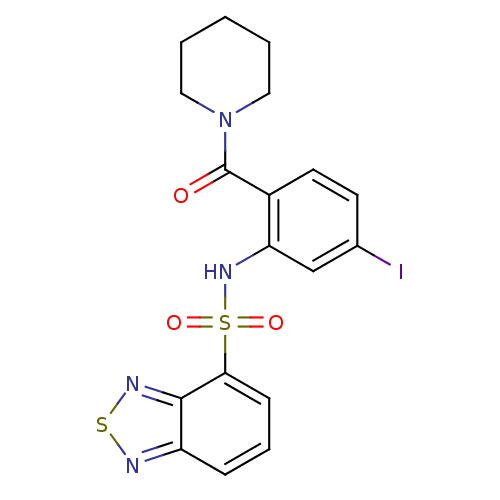
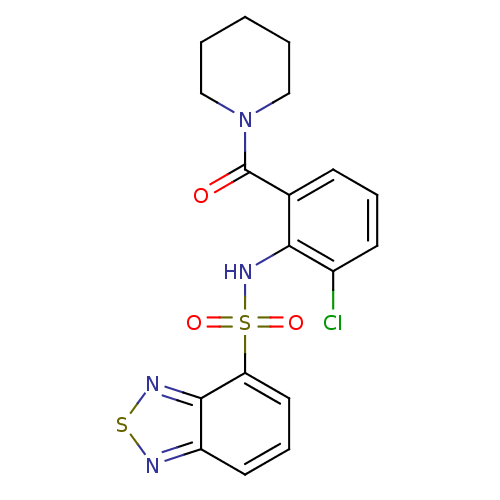
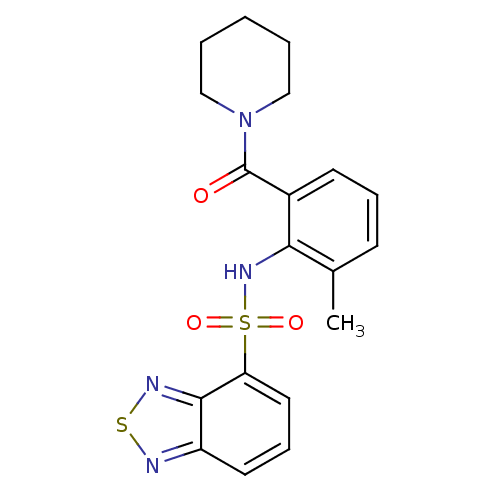
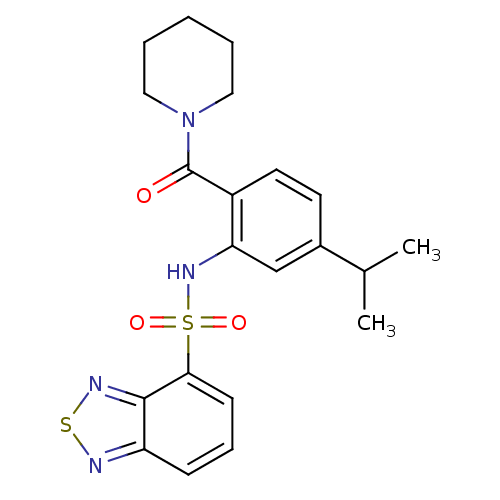
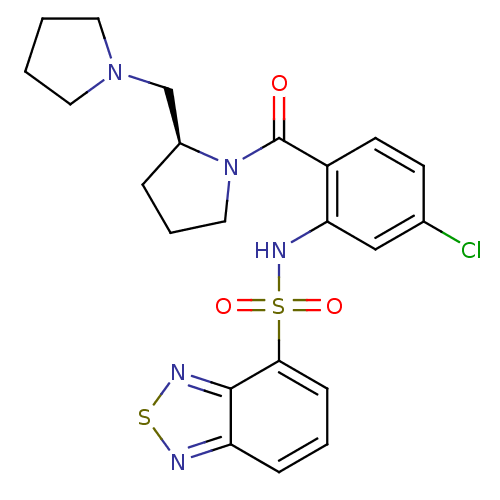
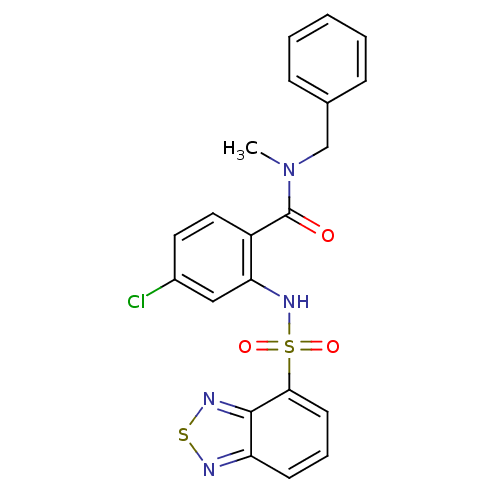
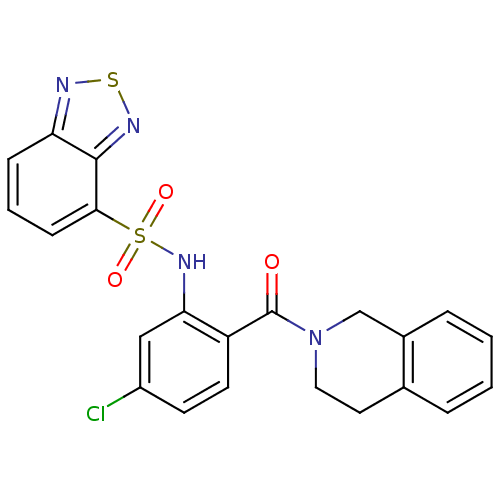
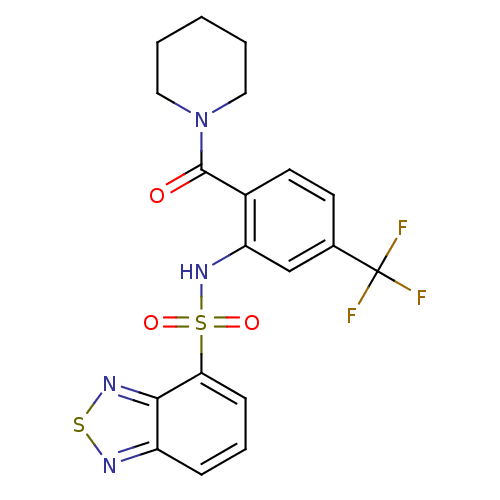
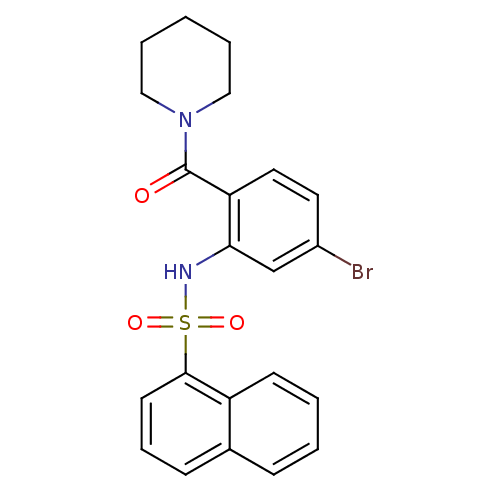
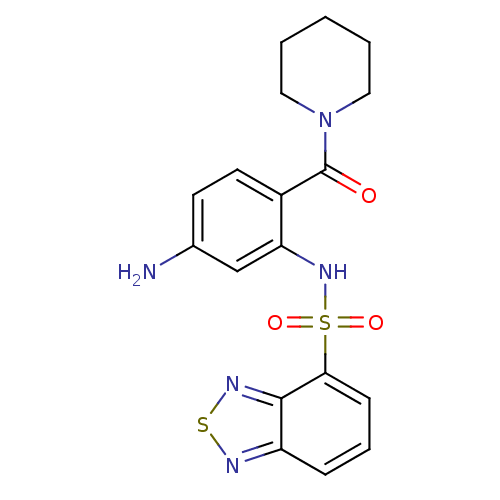
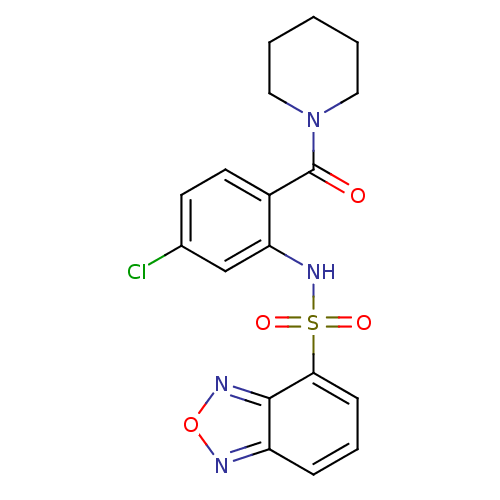
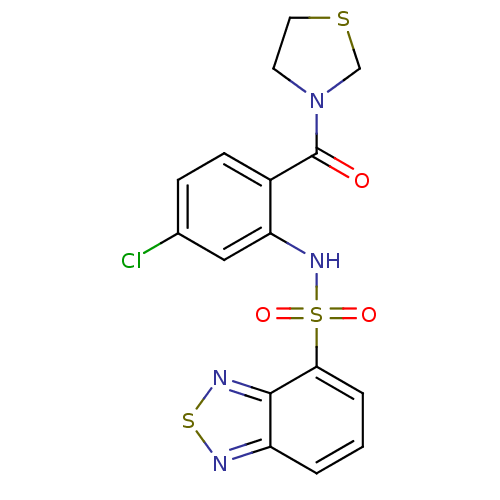
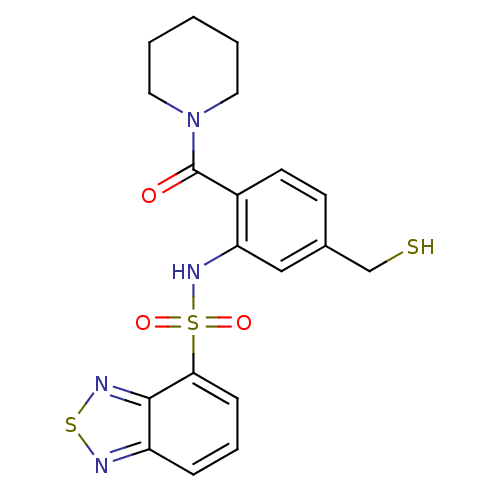
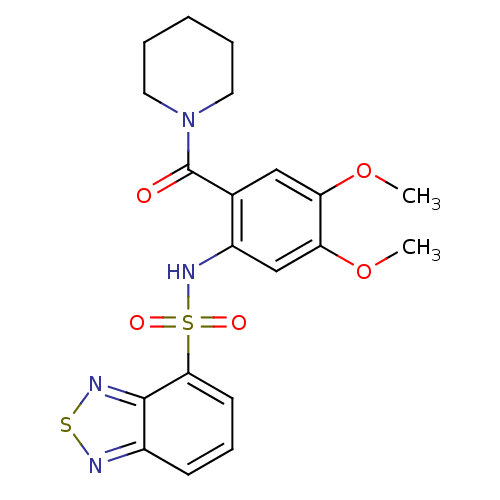
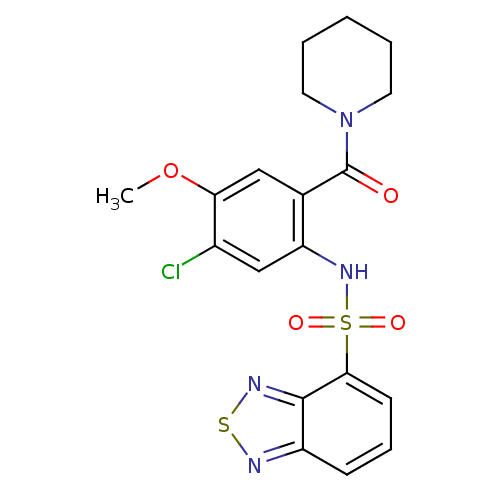
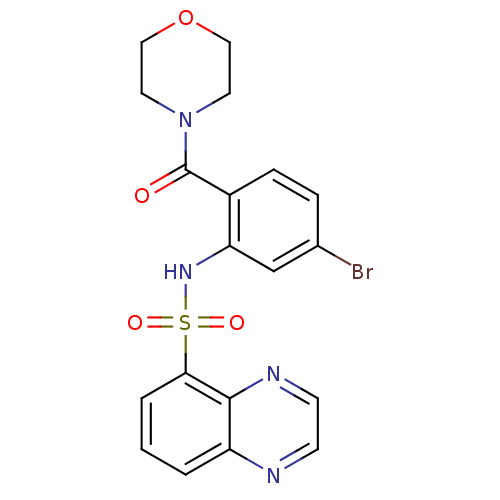
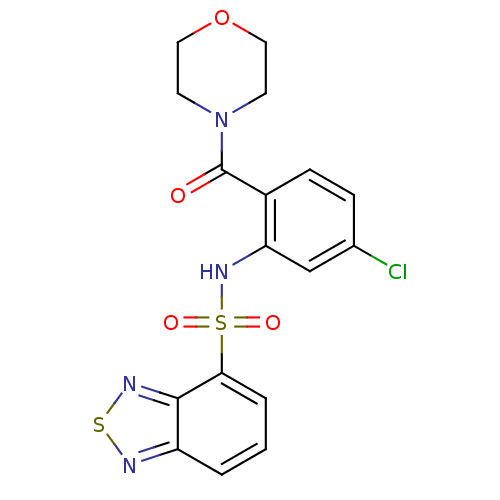
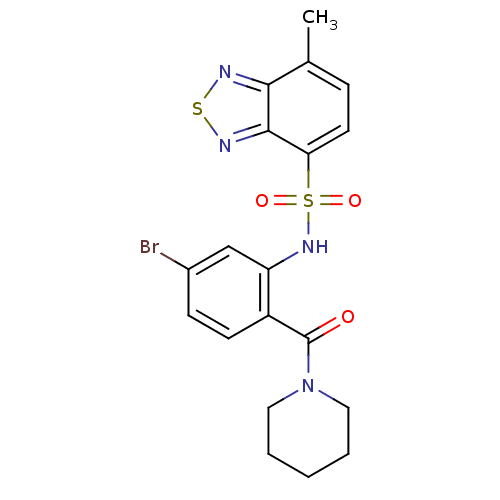
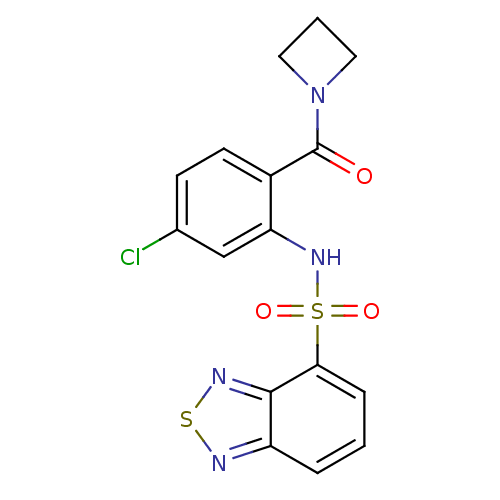
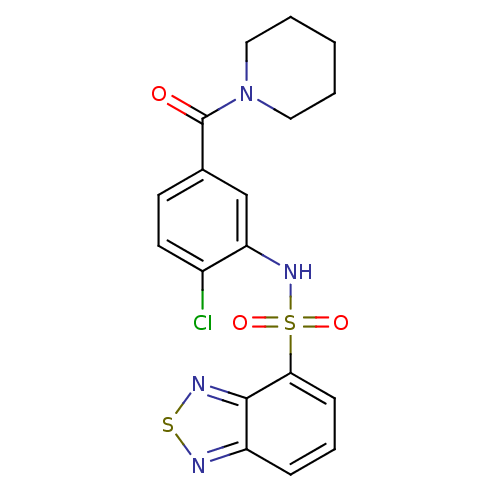
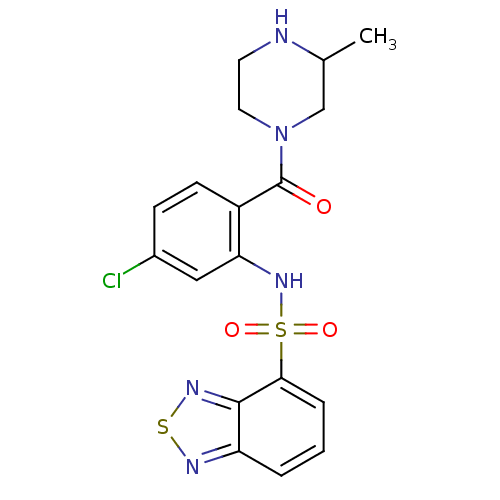
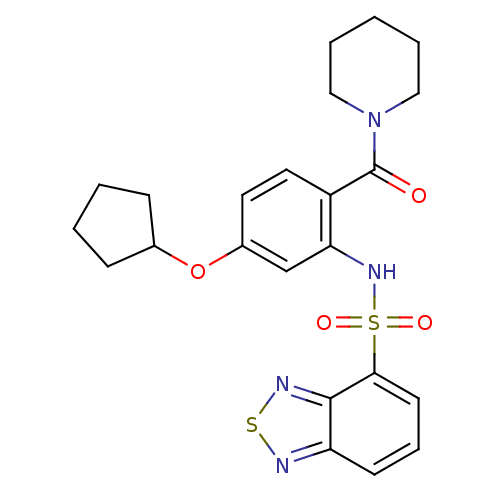
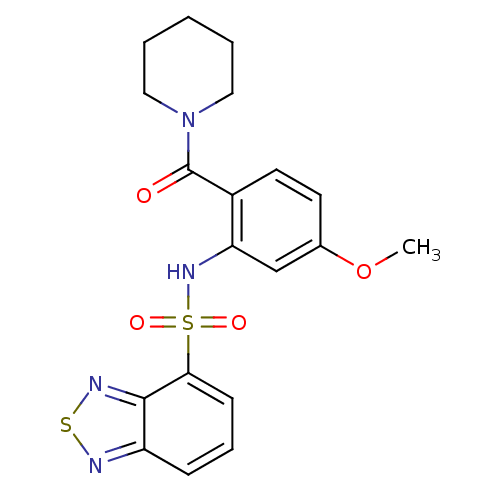
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK2RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK2RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK2RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK2RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK2RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK2RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK2RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK-8S from human CCK1RMore data for this Ligand-Target Pair