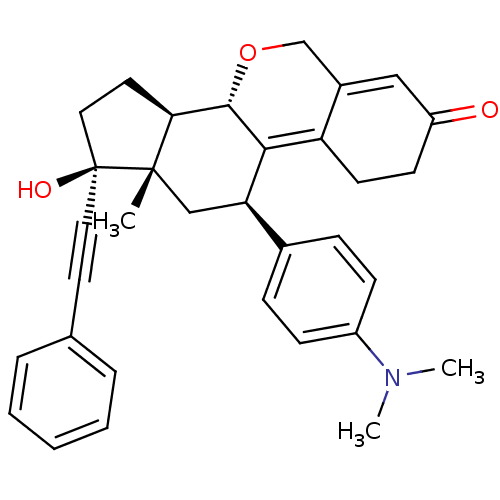
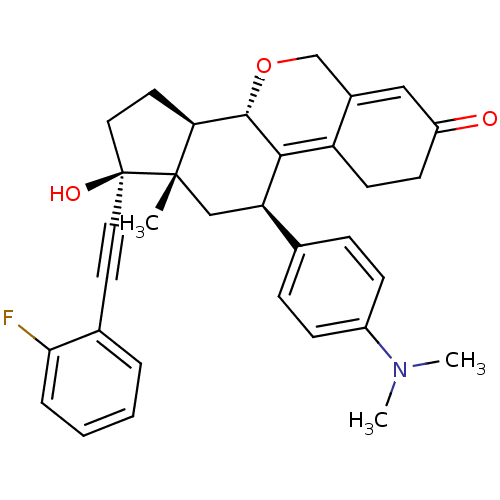
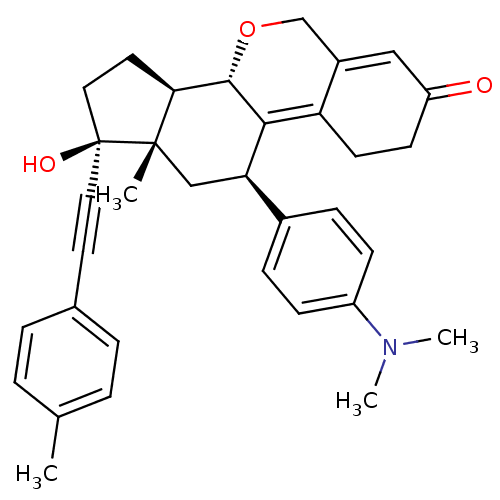
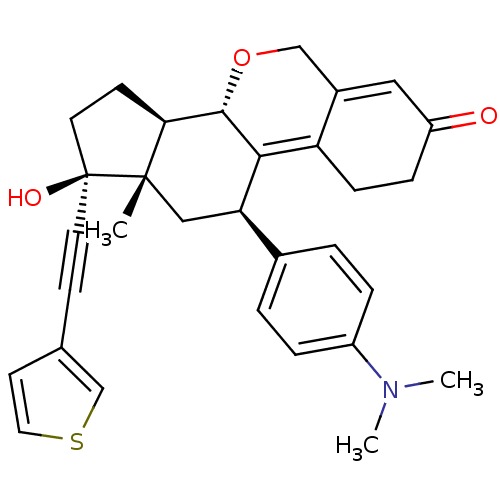
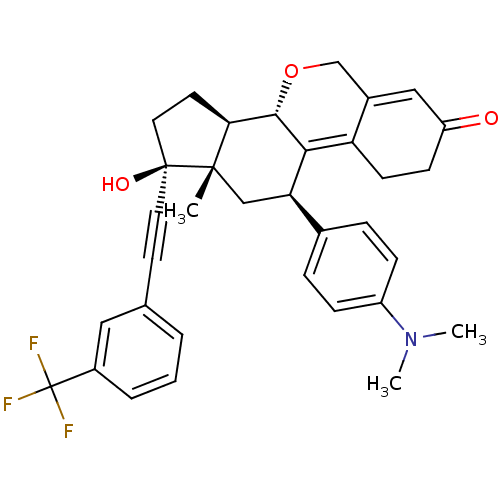
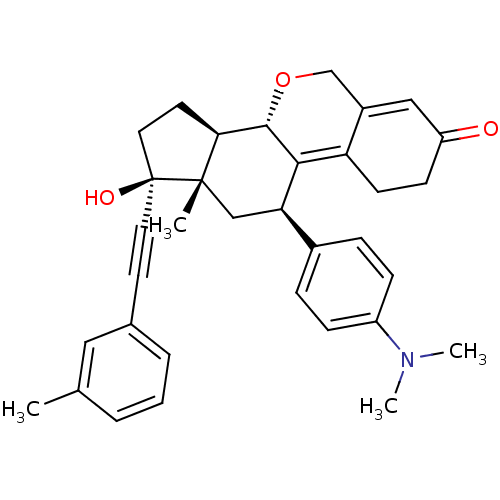
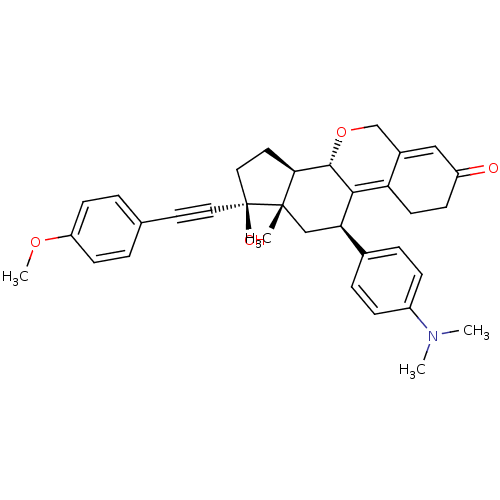
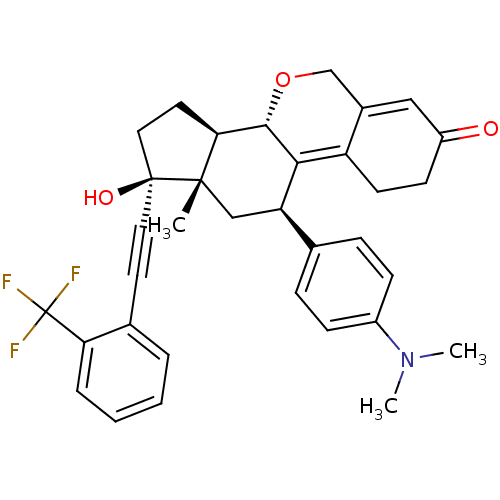
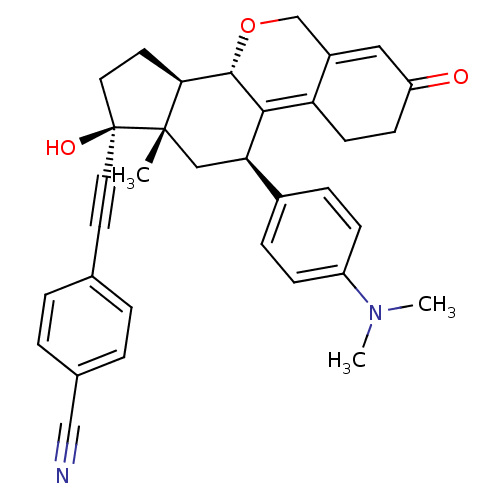
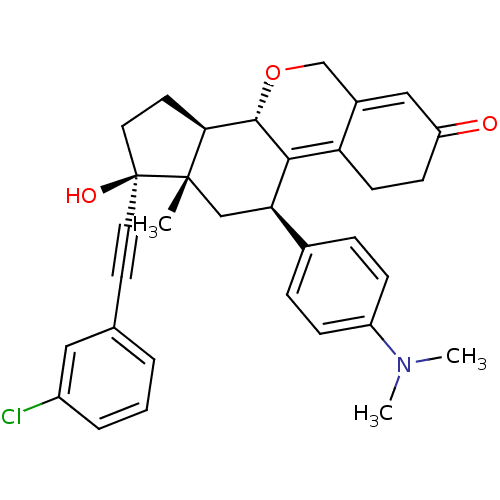
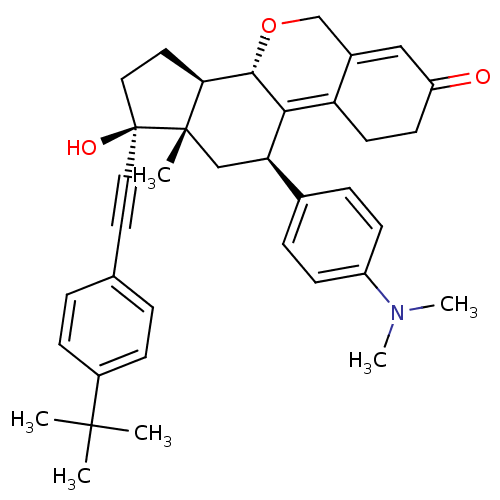
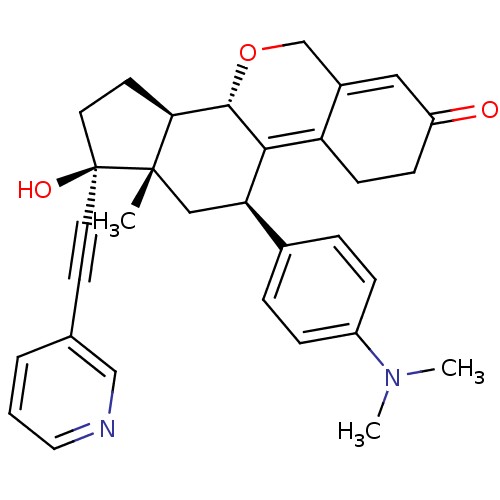
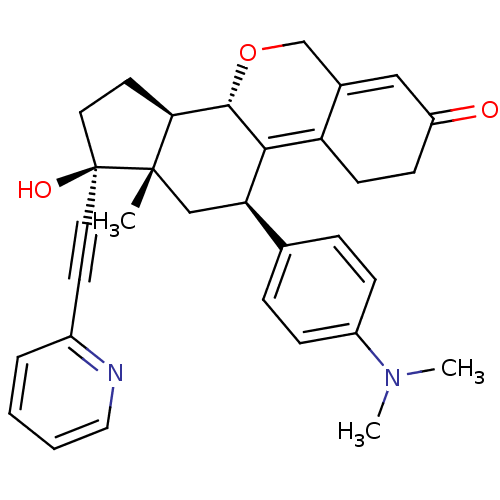
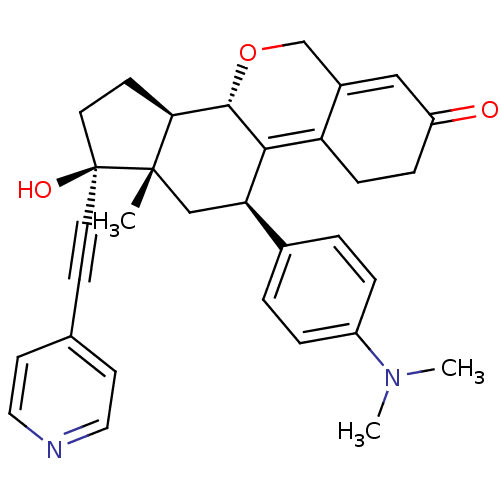
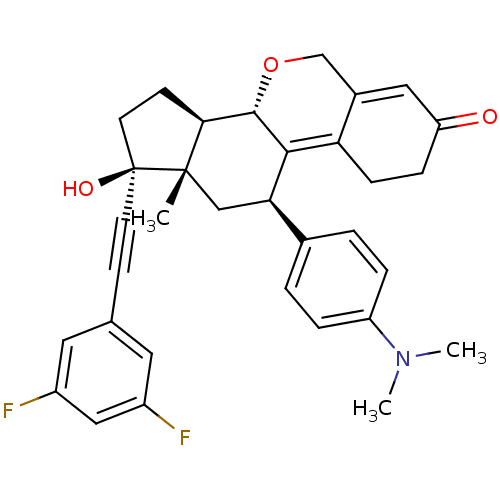
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.270nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
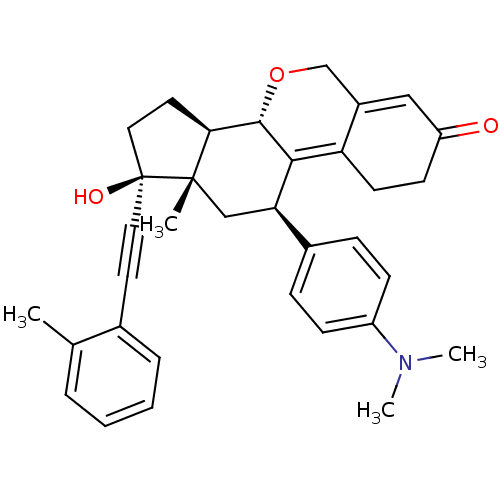
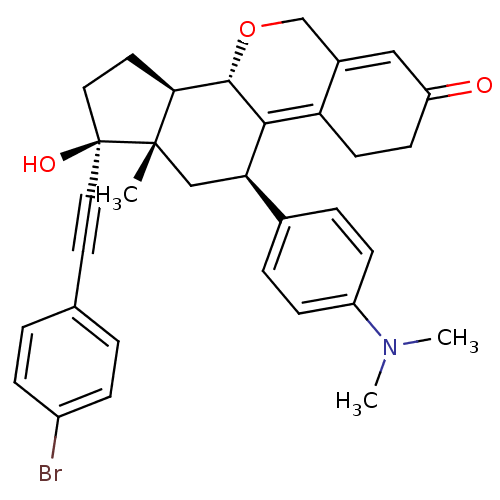
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.610nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
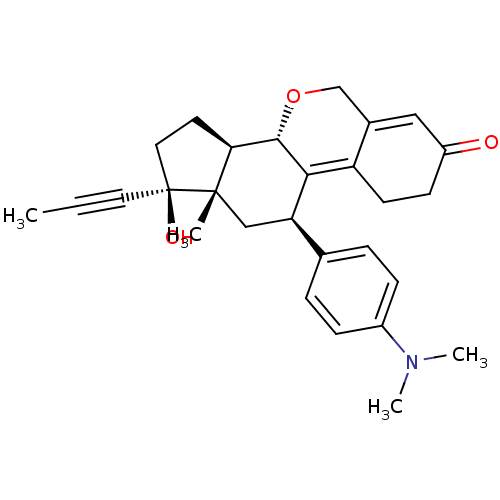
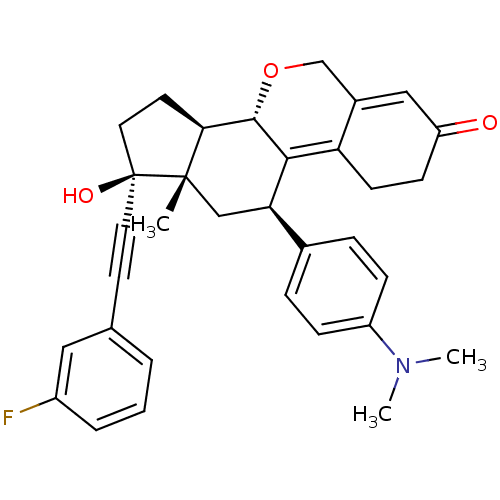
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.75nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
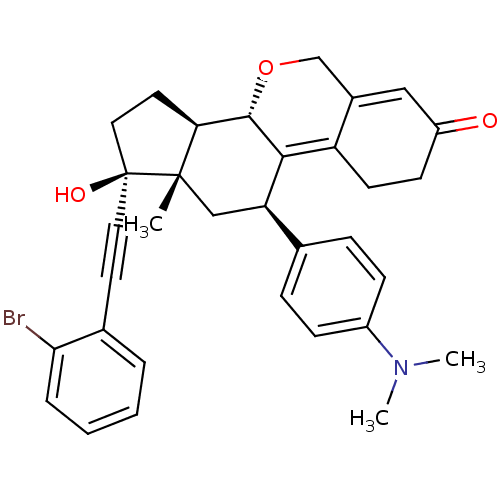
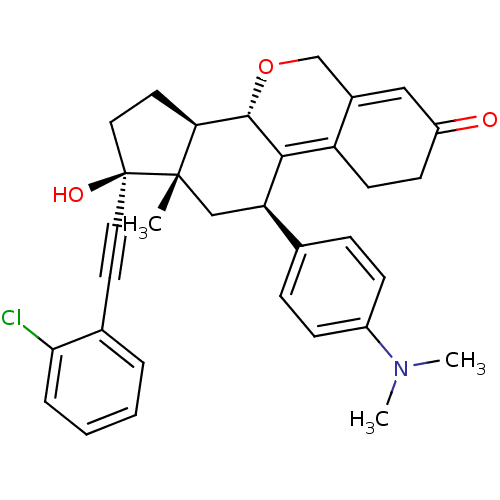
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.910nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 0.940nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 3.70nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 5.80nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 7.5nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 8.60nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 12.5nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 19.5nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 22.1nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 31.3nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 34nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 34.4nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 36.3nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 37.6nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetProgesterone receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 39nMAssay Description:Antagonist activity at human progesterone receptor assessed as inhibition of alkaline phosphatase activity in human T47D cellsMore data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 41.3nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 43.5nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 45.4nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 48.9nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 51nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 56nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 56.6nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 62.7nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 64.8nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 65.1nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 65.8nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 80.3nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 86.5nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 111nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 113nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 175nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 247nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 304nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair
TargetGlucocorticoid receptor(Homo sapiens (Human))
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson And Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataIC50: 342nMAssay Description:Antagonist activity at human glucocorticoid receptor assessed as inhibition of corticoid-induced transcription in human A549 cells by GRE-linked luci...More data for this Ligand-Target Pair

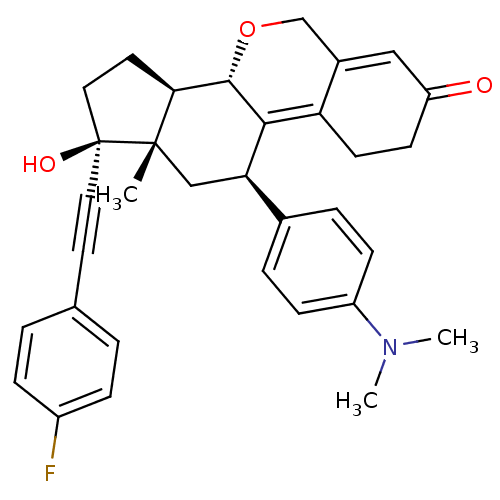
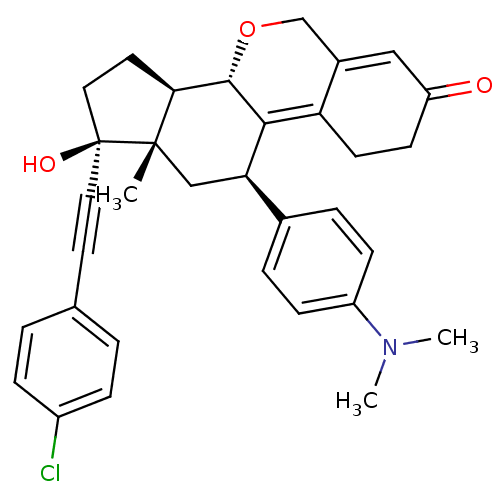
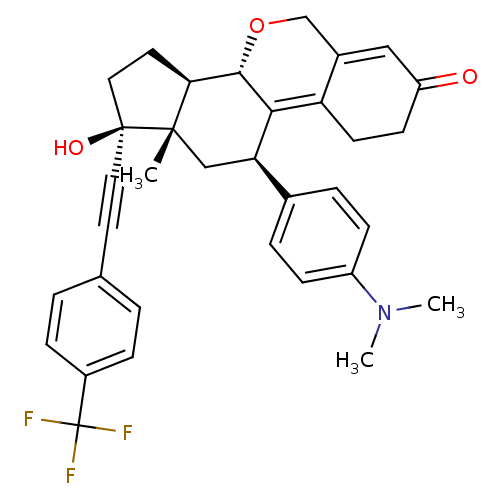

 3D Structure (crystal)
3D Structure (crystal)