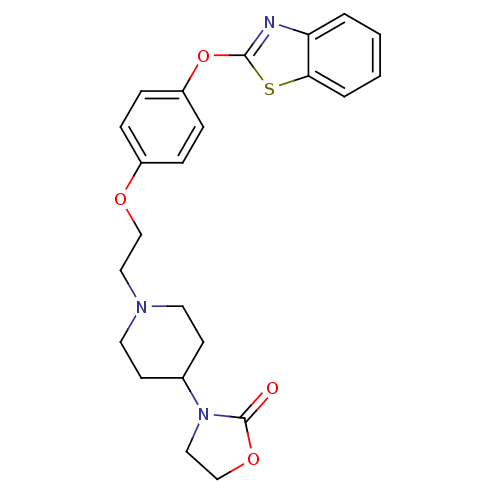
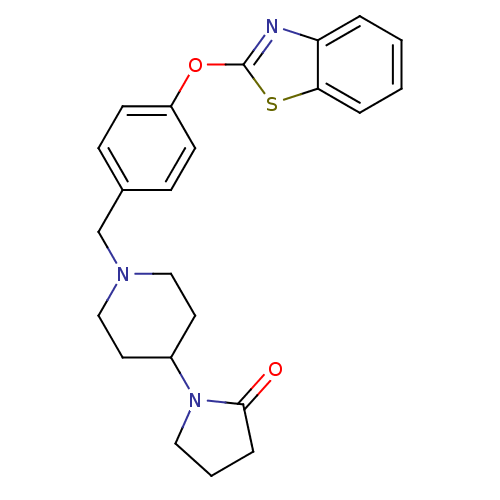
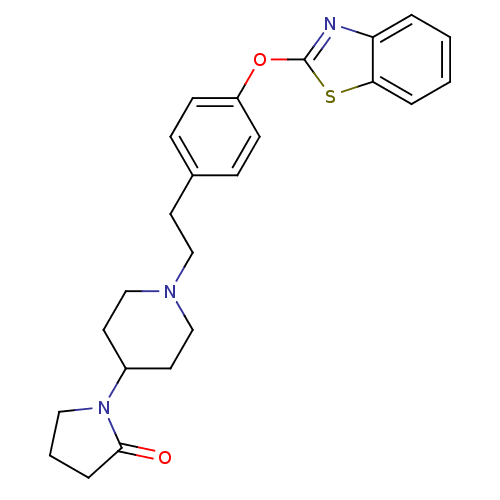
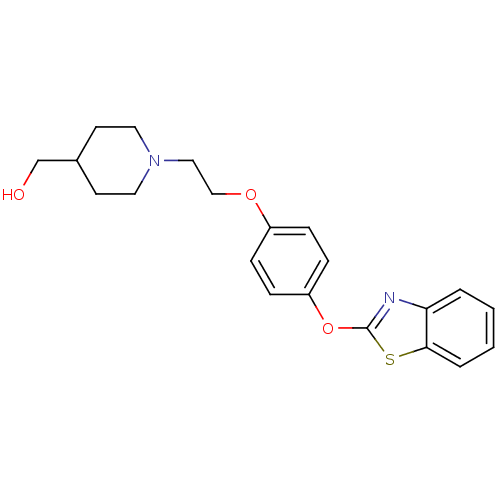
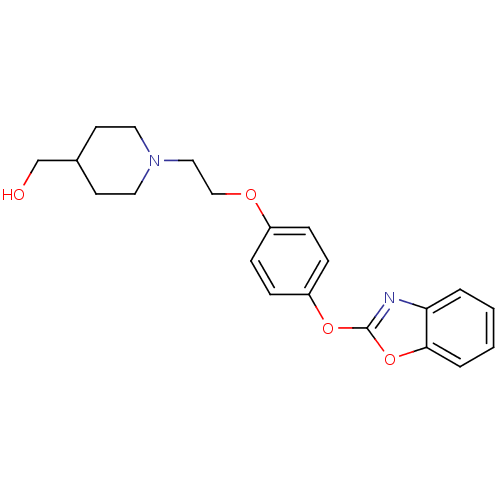
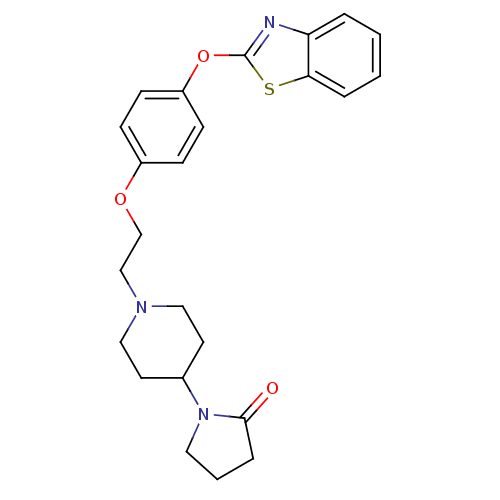
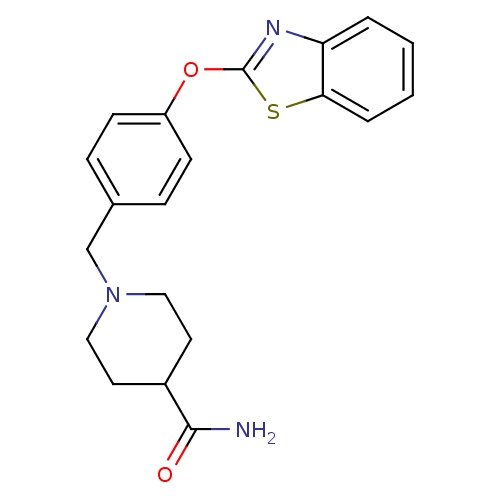
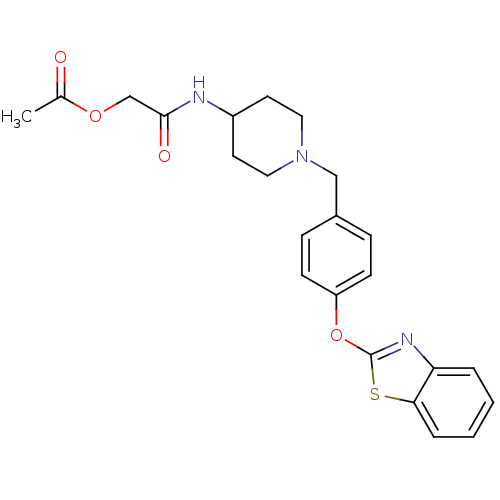
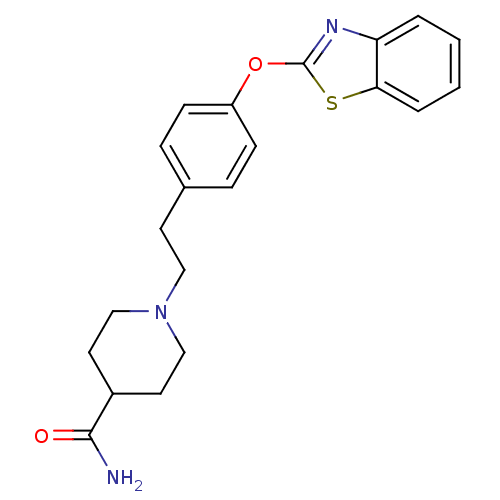
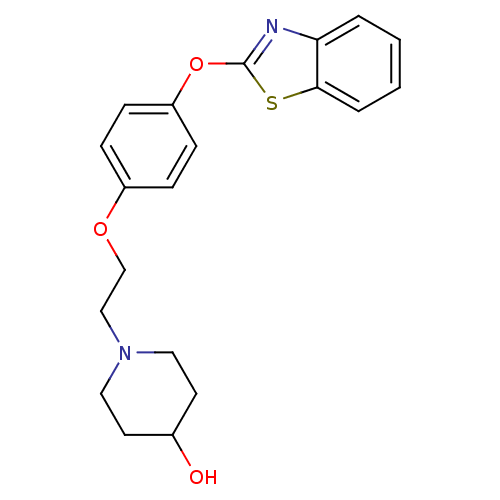
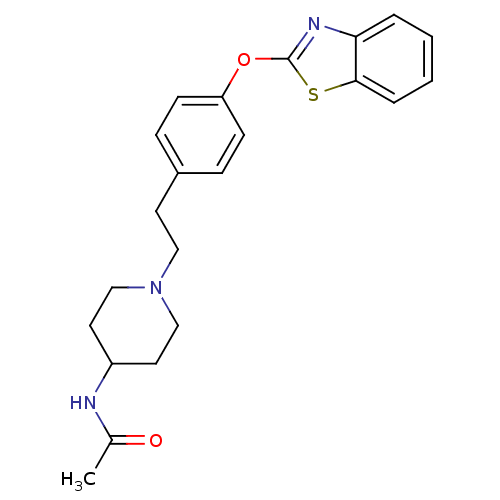
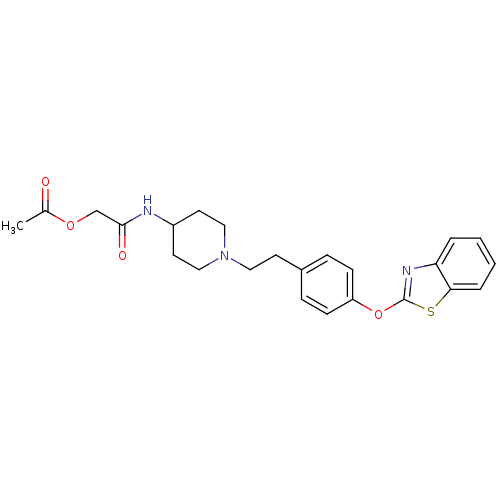
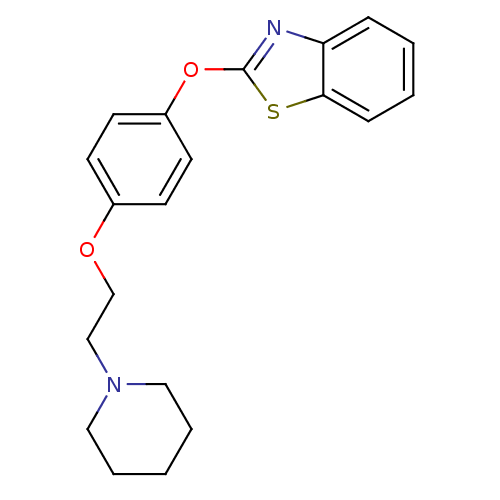
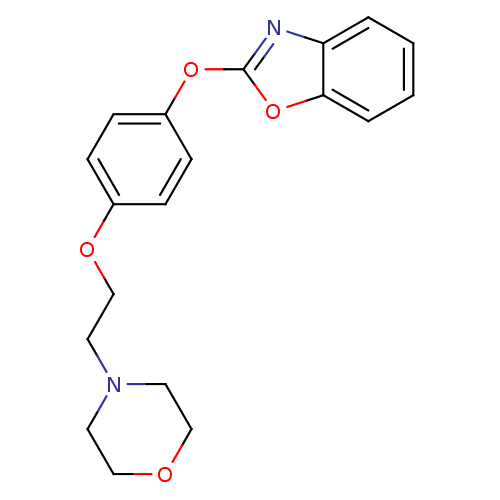
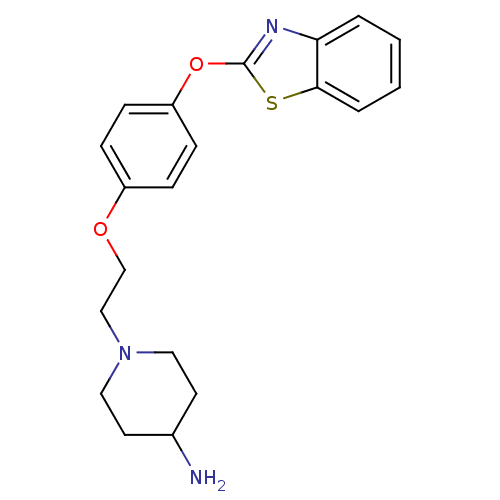
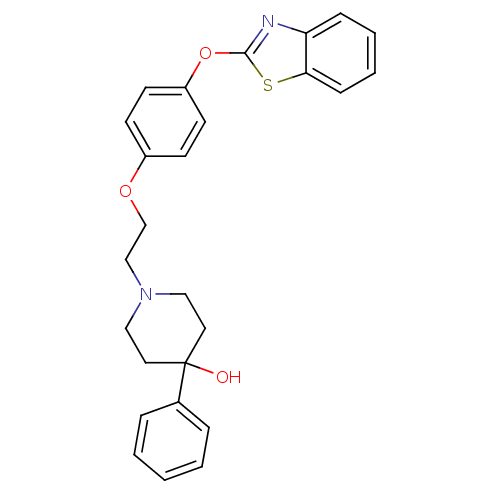
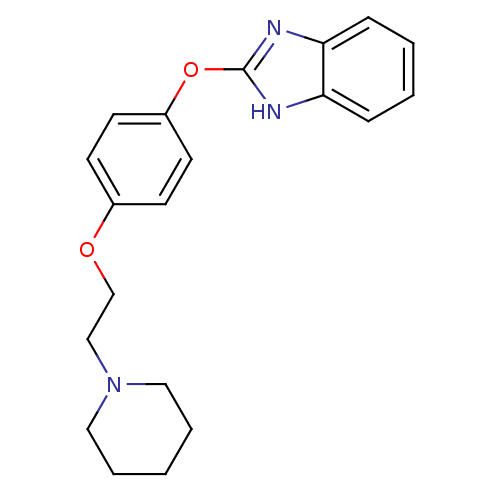
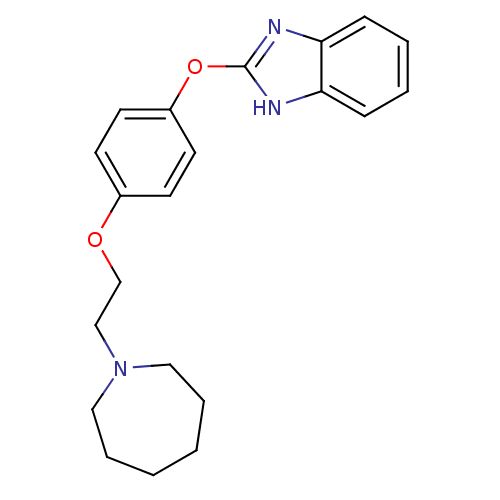
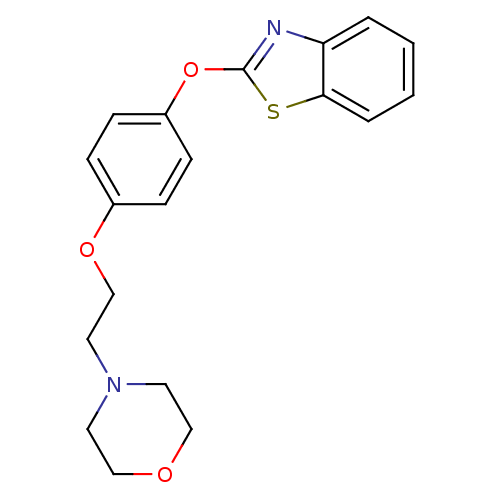
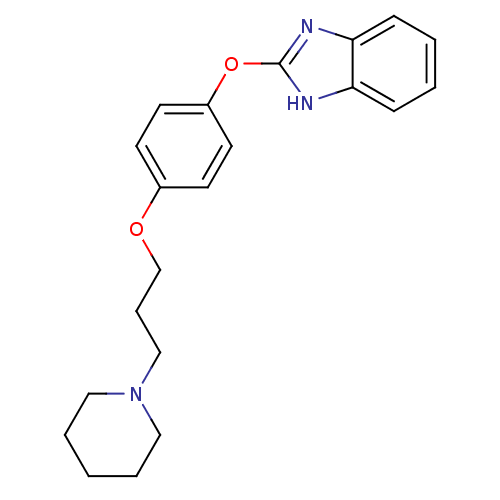
Affinity DataIC50: 4nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 9nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 31nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 35nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 54nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 58nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 59nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 66nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 66nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 66nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 70nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 84nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 87nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 110nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 140nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
Affinity DataIC50: 350nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataIC50: 500nMAssay Description:Compounds were assessed for their ability to displace [3H]astemizole using membranes from HEK-293 cells expressing the hERG K+ channel.More data for this Ligand-Target Pair
Affinity DataIC50: 710nMT: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataIC50: 1.00E+3nMpH: 7.4 T: 2°CAssay Description:Compounds were assessed for their ability to displace [3H]astemizole using membranes from HEK-293 cells expressing the hERG K+ channel.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataIC50: 1.00E+3nMAssay Description:Compounds were assessed for their ability to displace [3H]astemizole using membranes from HEK-293 cells expressing the hERG K+ channel.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataIC50: 1.80E+3nMT: 2°CAssay Description:Compounds were assessed for their ability to displace [3H]astemizole using membranes from HEK-293 cells expressing the hERG K+ channel.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataIC50: 2.00E+3nMpH: 7.4 T: 2°CAssay Description:Compounds were assessed for their ability to displace [3H]astemizole using membranes from HEK-293 cells expressing the hERG K+ channel.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataIC50: 2.70E+3nMT: 2°CAssay Description:Compounds were assessed for their ability to displace [3H]astemizole using membranes from HEK-293 cells expressing the hERG K+ channel.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataIC50: 2.70E+3nMT: 2°CAssay Description:Compounds were assessed for their ability to displace [3H]astemizole using membranes from HEK-293 cells expressing the hERG K+ channel.More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMpH: 7.4 T: 2°CAssay Description:Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical
Johnson & Johnson Pharmaceutical
Affinity DataIC50: 3.00E+3nMpH: 7.4 T: 2°CAssay Description:Compounds were assessed for their ability to displace [3H]astemizole using membranes from HEK-293 cells expressing the hERG K+ channel.More data for this Ligand-Target Pair


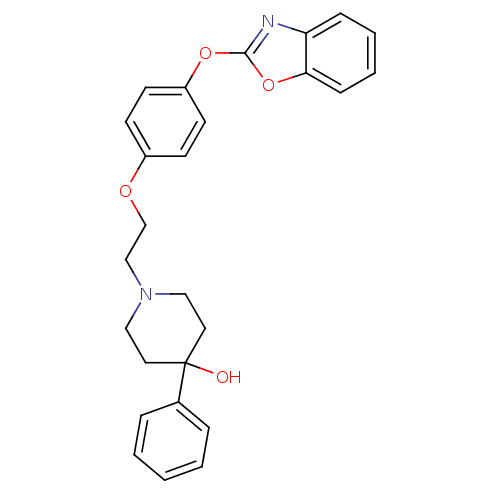


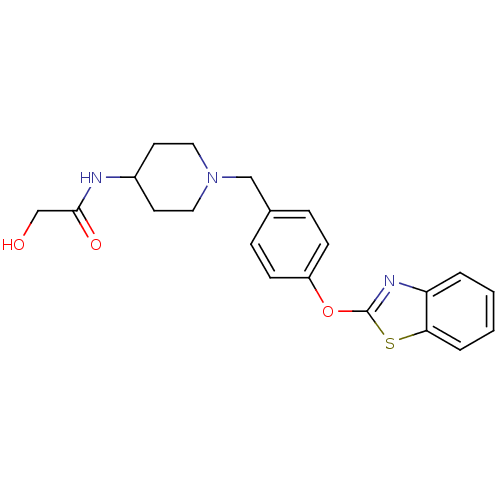
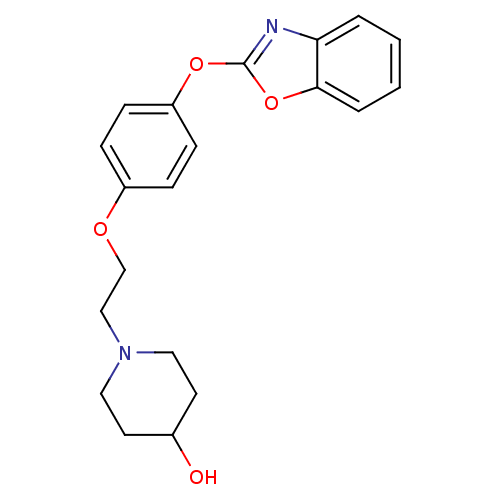
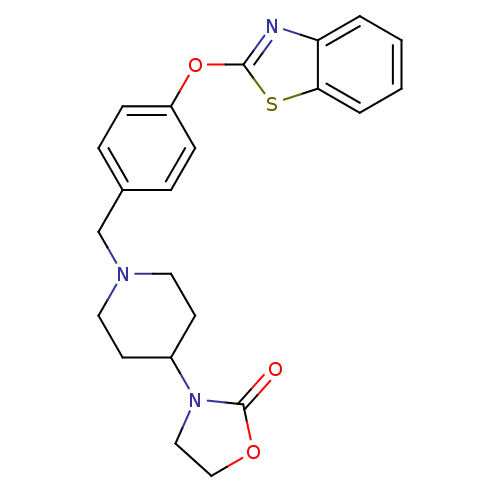
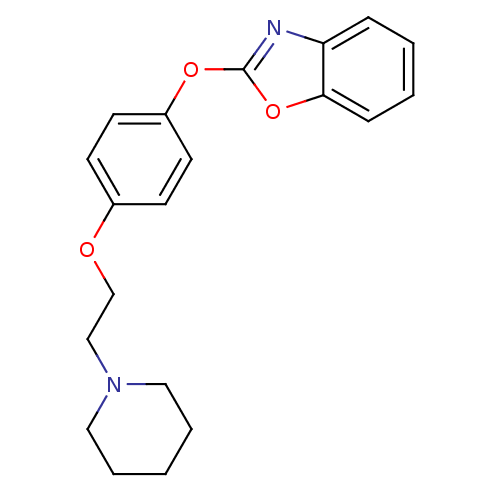
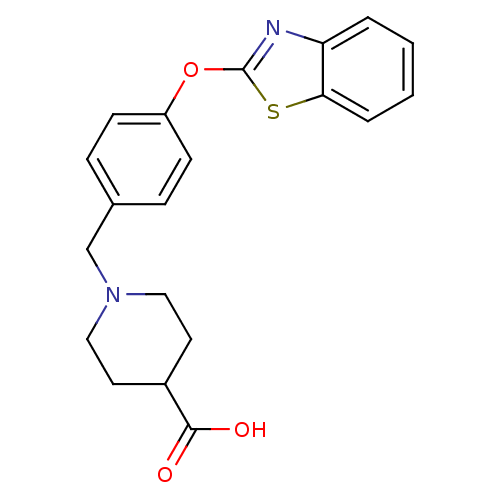
 3D Structure (crystal)
3D Structure (crystal)