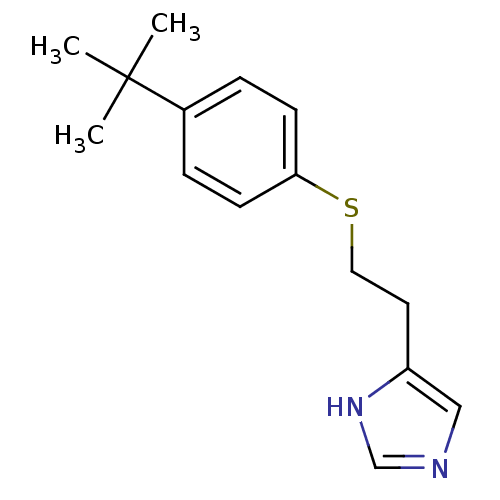
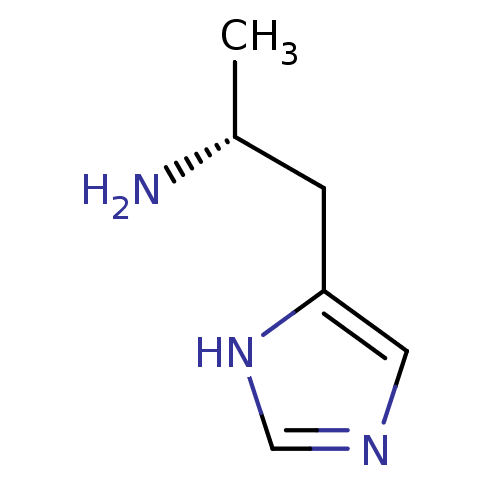
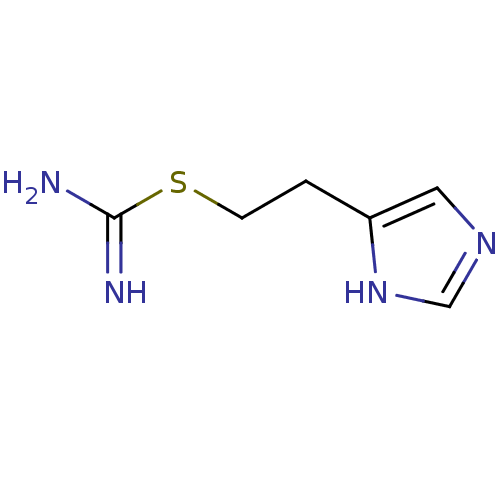
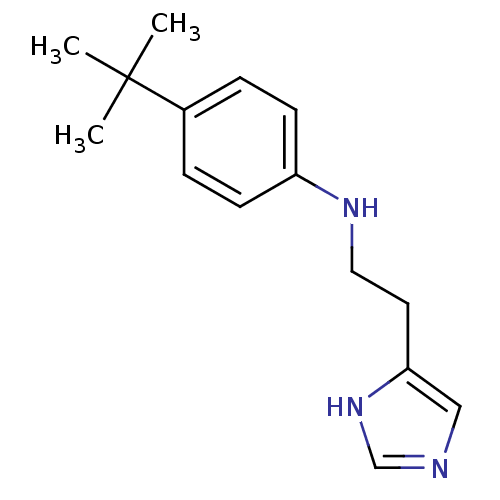
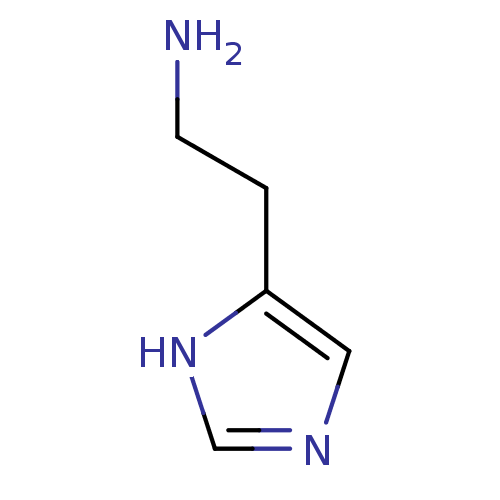
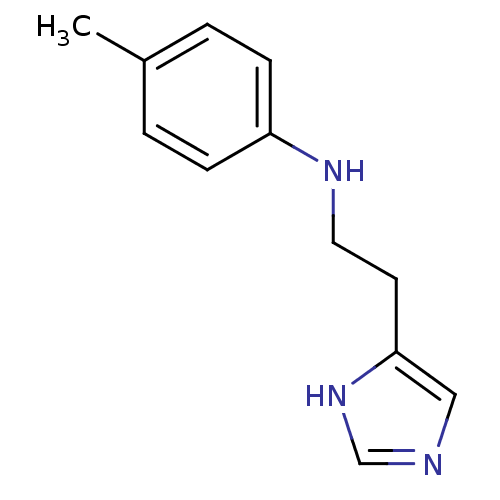
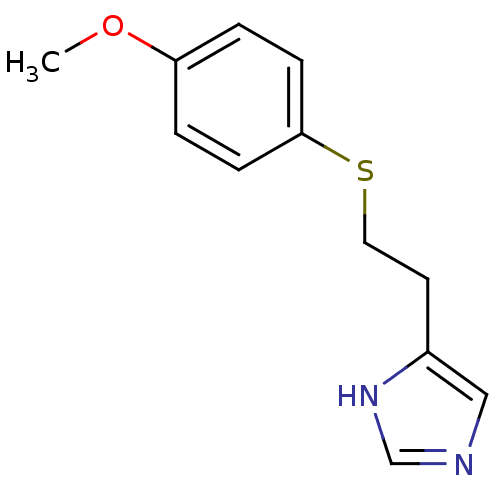
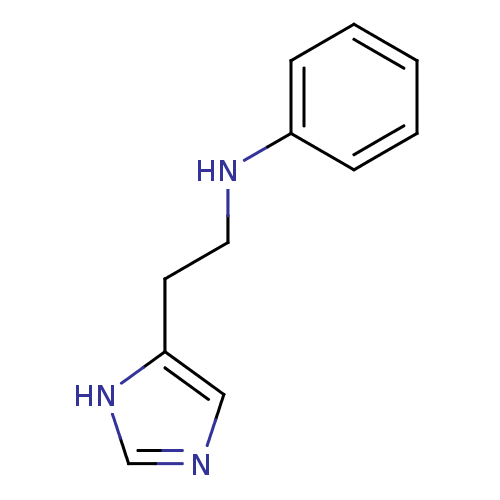
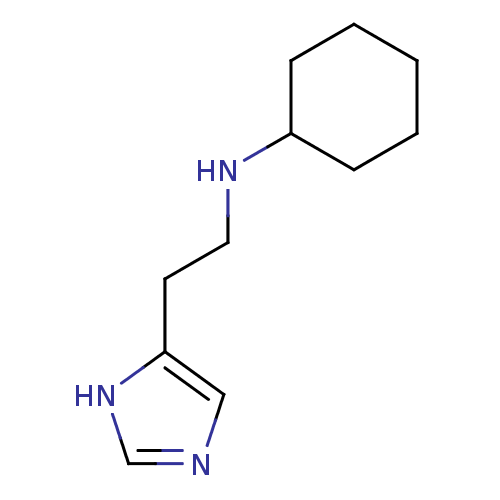
Affinity DataKi: 0.170nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.550nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.570nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 0.890nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human recombinant histamine H4 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 4.10nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 9.90nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: 56nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of beta-1 adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of dopamine D3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of beta2 adrenergic receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of muscarinic M1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of muscarinic M3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of 5HT1A receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of dopamine D2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of 5HT3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of 5HT2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of dopamine D1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of histamine H2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of histamine H1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+3nMAssay Description:Displacement of [3H[N-alpha-methylhistamine form human recombinant histamine H3 receptor expressed in CHO cells after 1 hr by liquid scintillation co...More data for this Ligand-Target Pair
Affinity DataIC50: 8.5nMAssay Description:Inhibition of human CYP2C19 expressed in insect cell microsome after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 470nMAssay Description:Inhibition of human CYP3A4 expressed in insect cell microsome after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 680nMAssay Description:Inhibition of human CYP1A2 expressed in insect cell microsome after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 680nMAssay Description:Inhibition of human CYP2D6 expressed in insect cell microsome after 30 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.80nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 88nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 25nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 1.60nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 4.60nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 19nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 0.75nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 3.40E+3nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 2.40nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 65nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 640nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 320nMAssay Description:Agonist activity at human recombinant histamine H3 receptor expressed in CHO cells assessed as [35S]GTPgammaS binding after 1 hr by liquid scintillat...More data for this Ligand-Target Pair