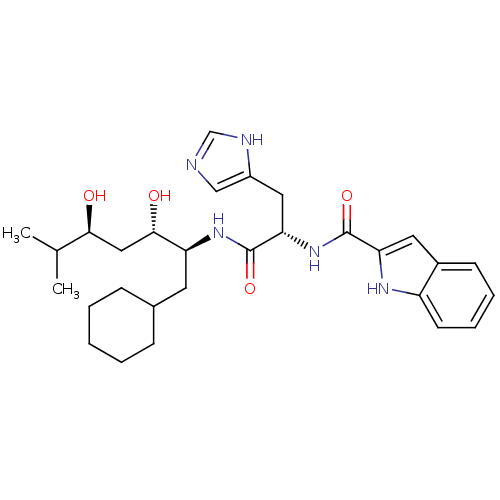
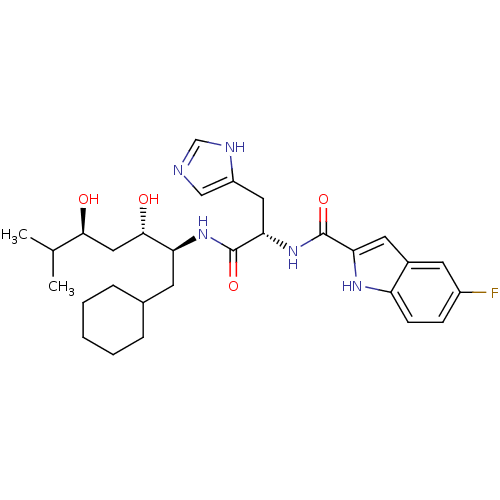
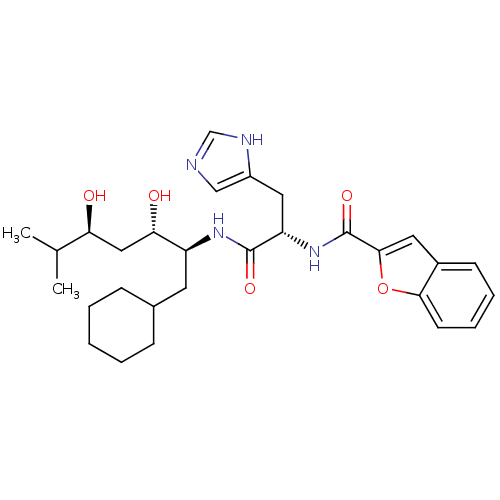
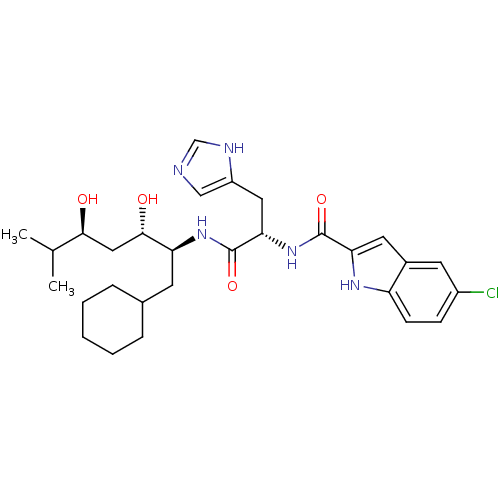
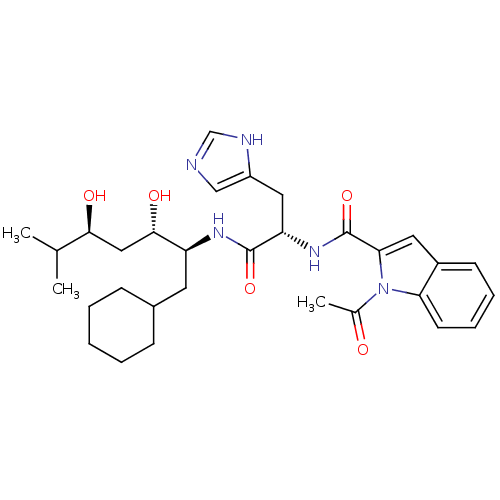
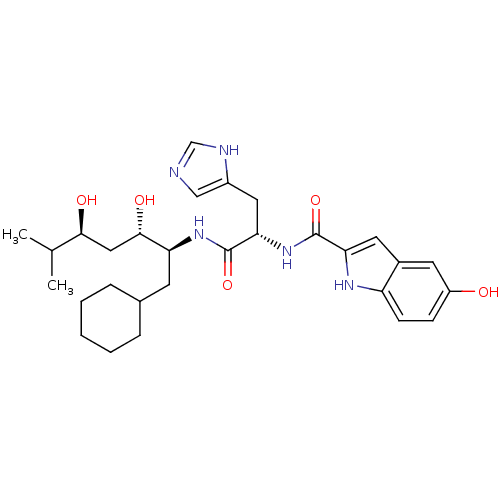
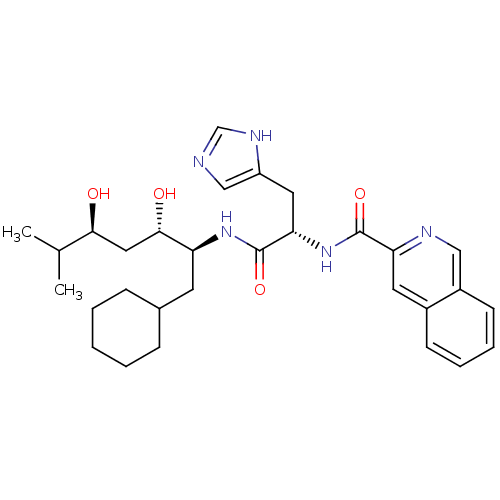
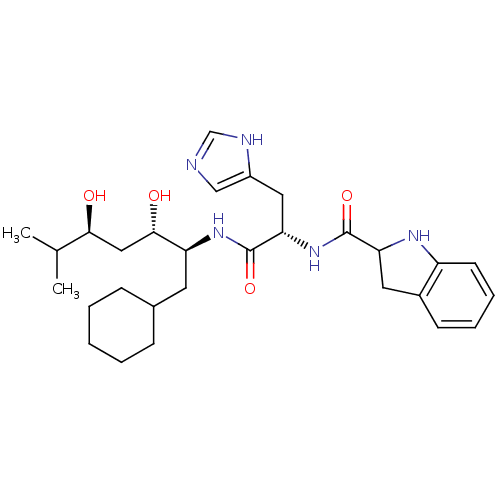
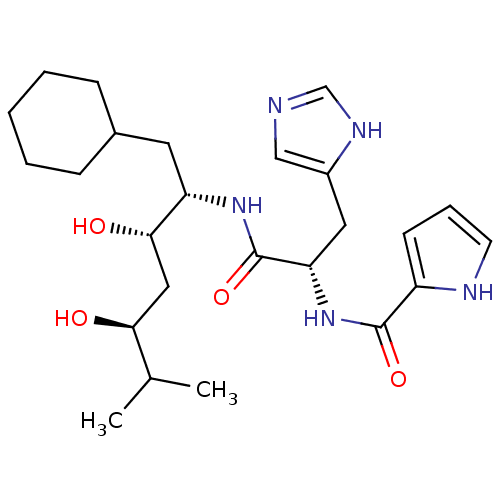
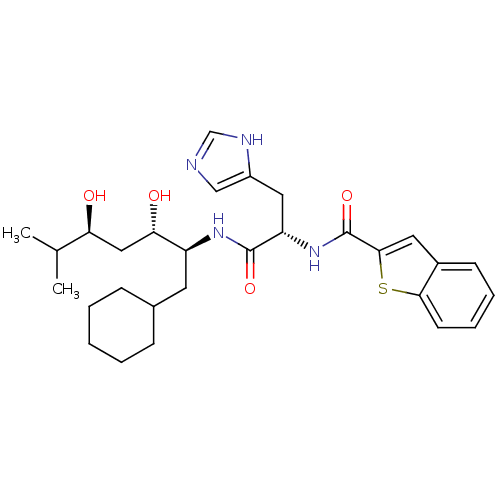
Affinity DataIC50: 0.680nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:In vitro renin inhibitory effect of the compound was evaluated for plasma renin activity (PRA) of marmoset plasma renin, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 93nMAssay Description:In vitro renin inhibitory effect of the compound was evaluated for plasma renin activity (PRA) of dog plasma renin, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 260nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 340nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 420nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 790nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 880nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 880nMAssay Description:In vitro renin inhibitory effect of the compound was evaluated for plasma renin activity (PRA) of monkey plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+3nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: 8.20E+3nMAssay Description:In vitro renin inhibitory effect was evaluated for plasma renin activity (PRA) of human plasma, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory activity of the compound against cathepsin D (bovine), expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibitory activity of the compound against ACE human, expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:In vitro inhibitory effect of the compound was evaluated against pepsin, Expressed as IC50More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:In vitro renin inhibitory effect of the compound was evaluated for plasma renin activity (PRA) of rat plasma renin, Expressed as IC50More data for this Ligand-Target Pair