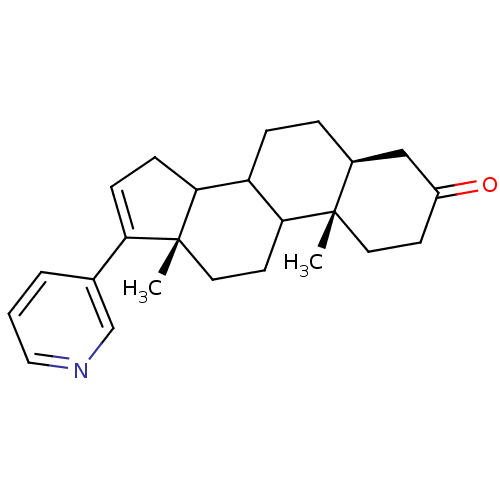
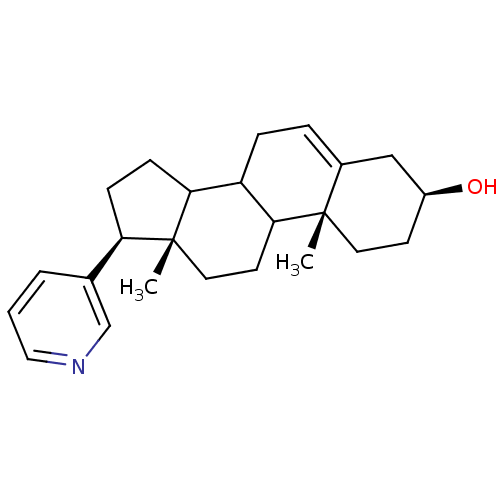
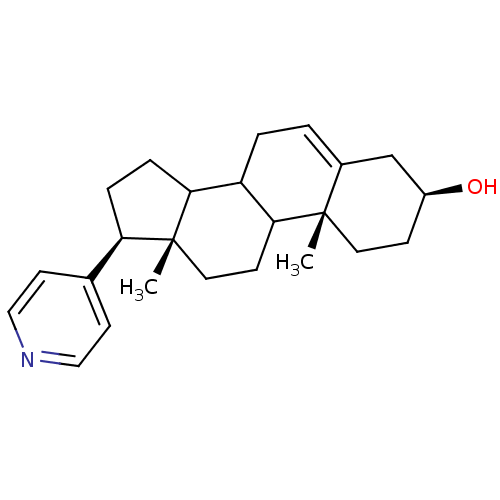
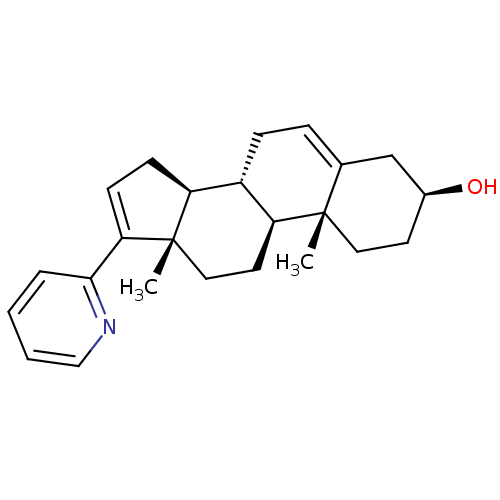
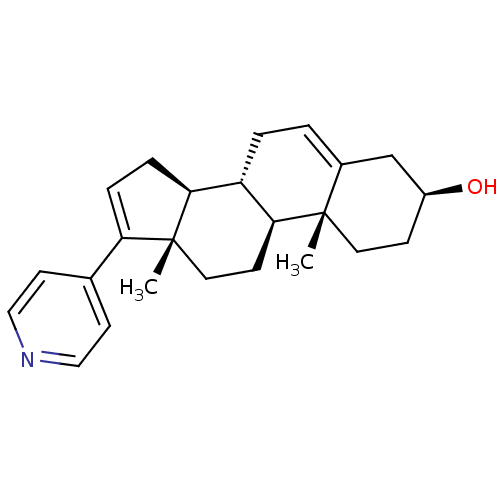
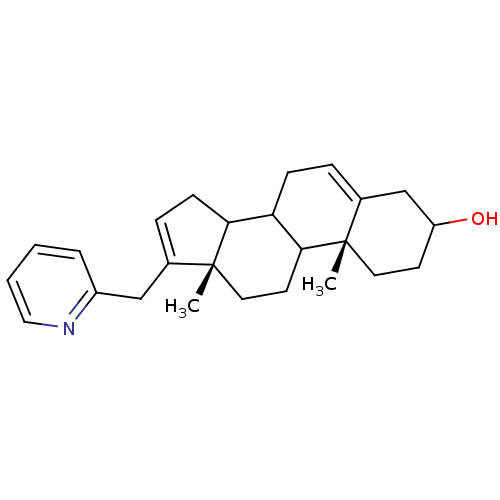
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 4.70nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 5.60nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 26nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 53nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 65nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 76nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of Cytochrome P450 19A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Ability to inhibit the C17,20-lyase enzyme by 50% using 17-alpha-hydroxyprogesterone as substrate.More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Institute Of Cancer Research
Curated by ChEMBL
Institute Of Cancer Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Ability to inhibit the Steroid 17-alpha-hydroxylase/17,20 lyase enzyme by 50%.More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of Cytochrome P450 19A1More data for this Ligand-Target Pair

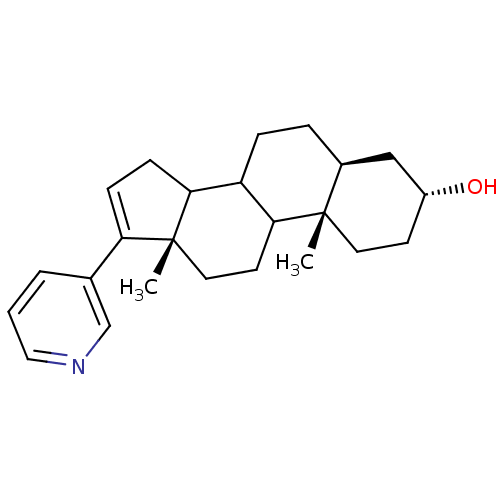
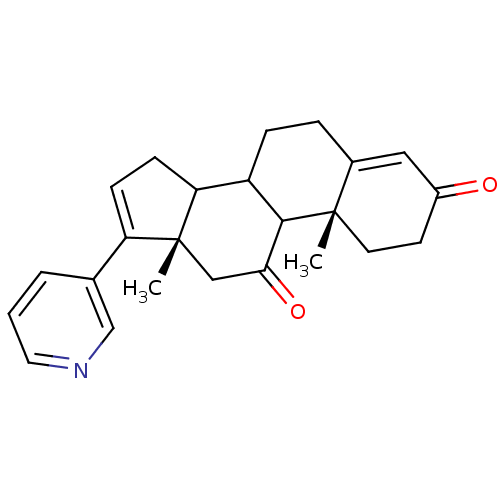
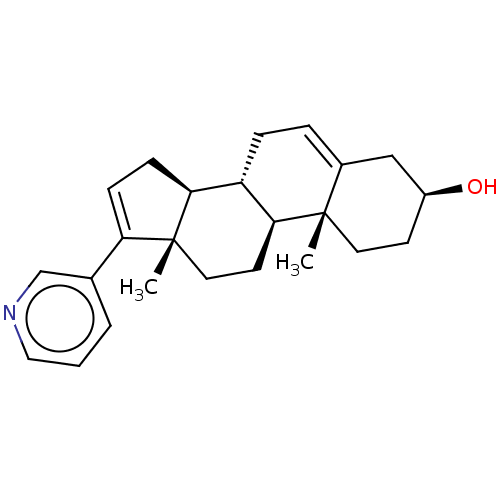
 3D Structure (crystal)
3D Structure (crystal)