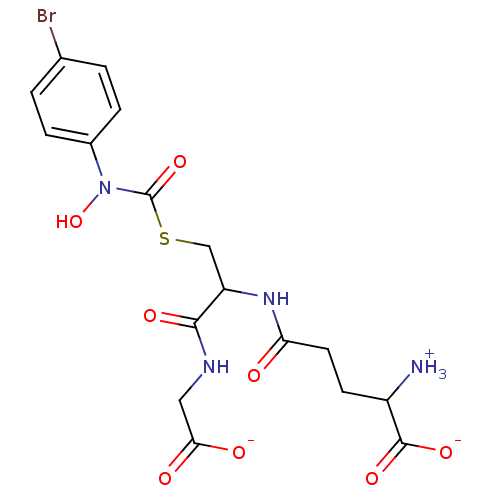
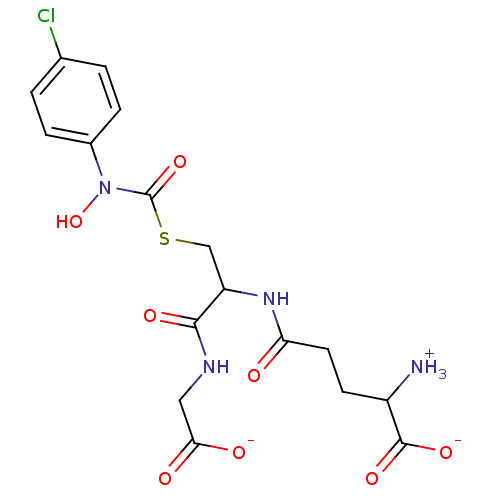
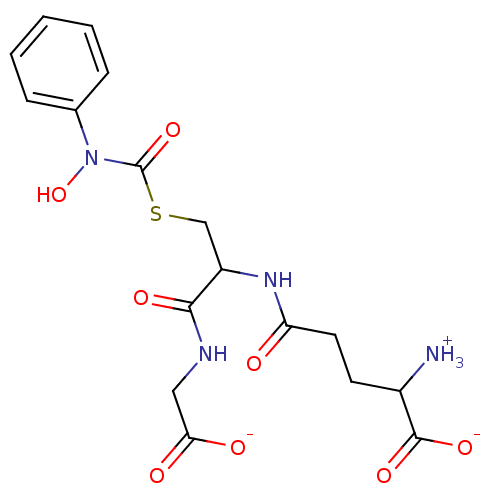
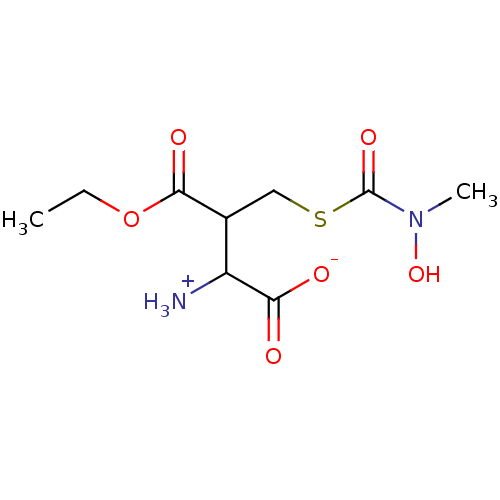
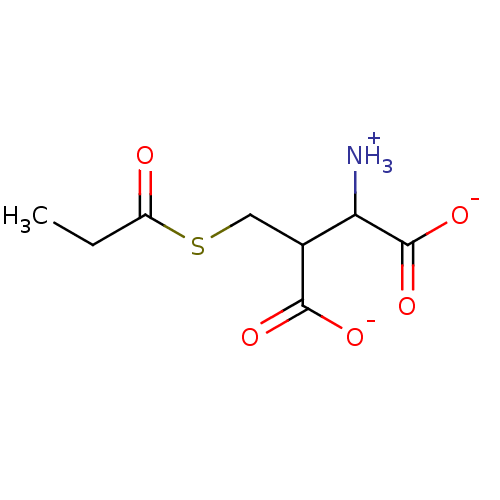
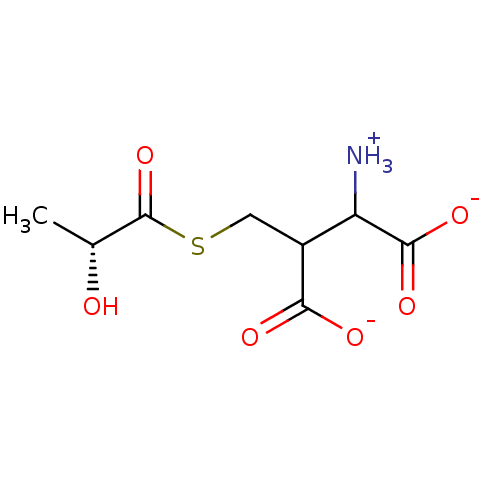
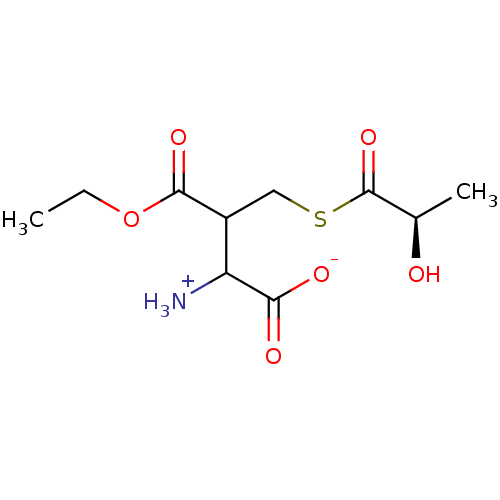
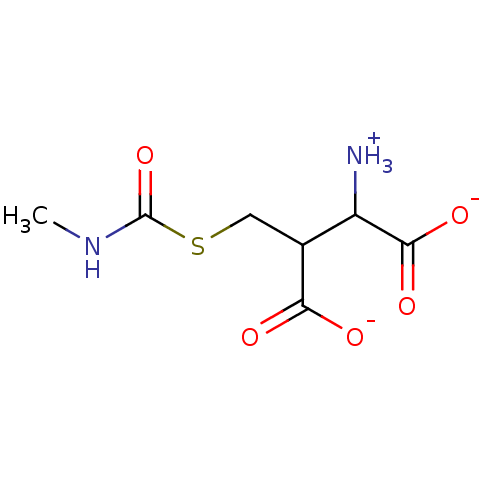
Affinity DataKi: 14nMAssay Description:Tested for inhibitory activity against human erythrocyte glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Tested for inhibitory activity against human erythrocyte glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Tested for inhibitory activity against human erythrocyte glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataKi: 1.20E+3nMAssay Description:Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nMAssay Description:Tested for inhibitory activity against human erythrocyte glyoxalase IMore data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataKi: 1.10E+4nMAssay Description:Inhibition constant for the inhibition of the hydrolysis of S-D-lactoylglutathione by glyoxalase IIMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+4nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
TargetHydroxyacylglutathione hydrolase, mitochondrial(Homo sapiens (Human))
University Of Maryland
Curated by ChEMBL
University Of Maryland
Curated by ChEMBL
Affinity DataKi: 4.26E+5nMAssay Description:Inhibition constants obtained from using the enediol analogs as competitive inhibitors for the inhibition of thehydrolysis of S-D-lactoylglutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 6.80E+5nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 1.98E+6nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 2.23E+6nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 3.16E+6nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair
Affinity DataKi: 5.67E+6nMAssay Description:Tested for inhibitory activity against yeast glyoxalase IMore data for this Ligand-Target Pair