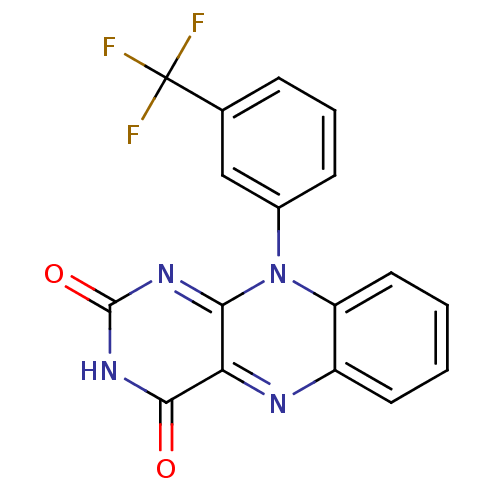
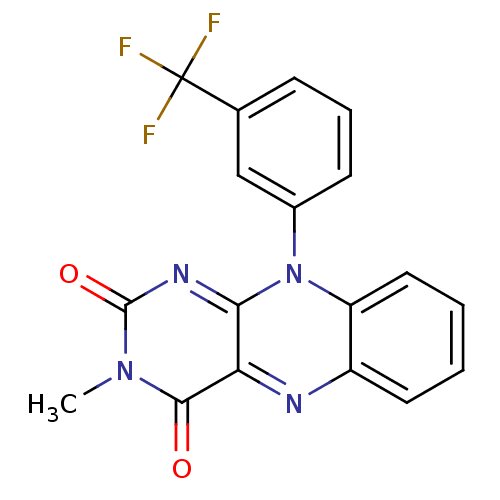
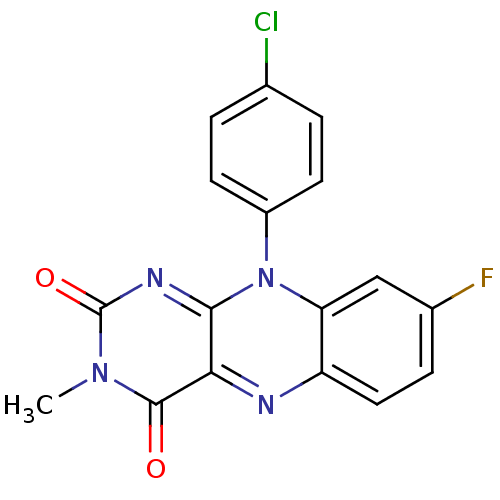
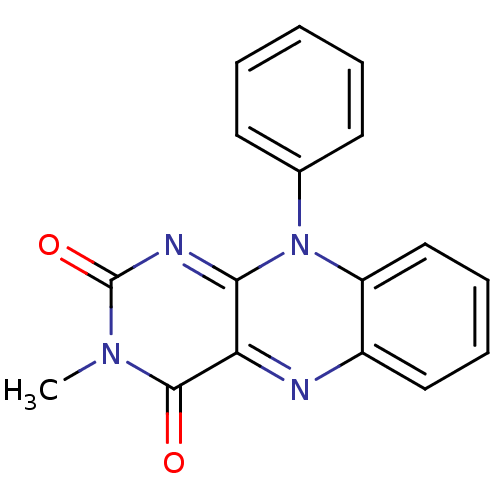
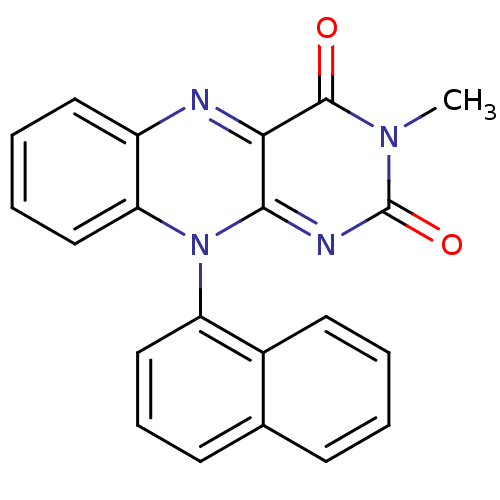
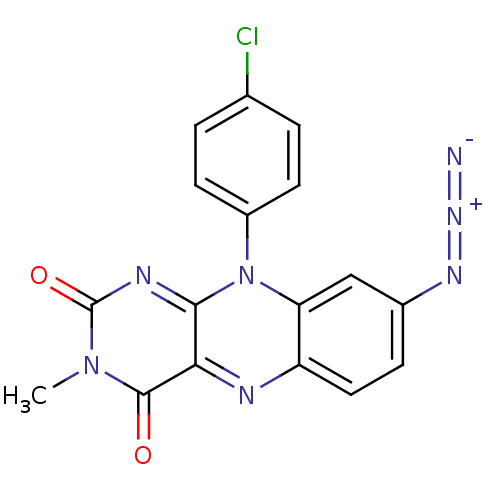
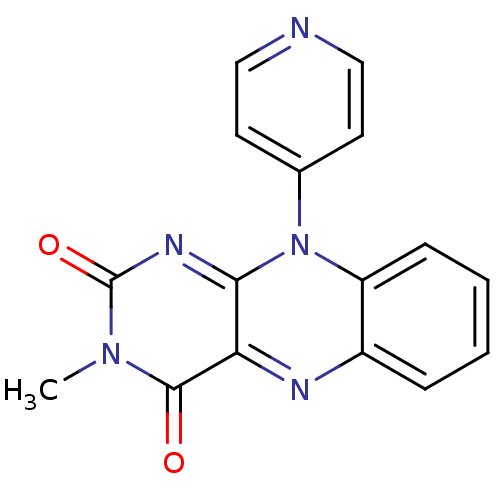
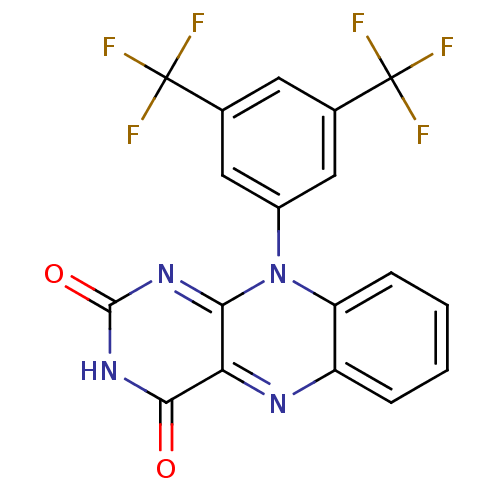
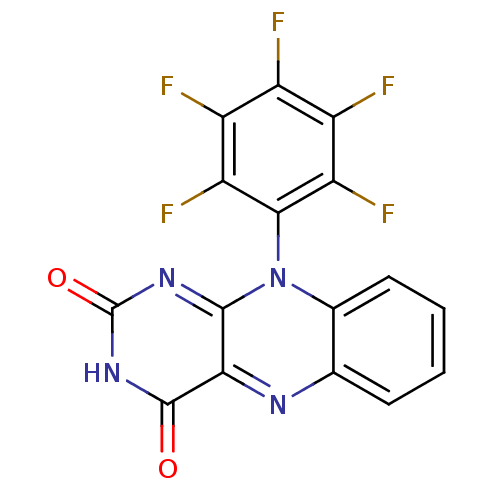
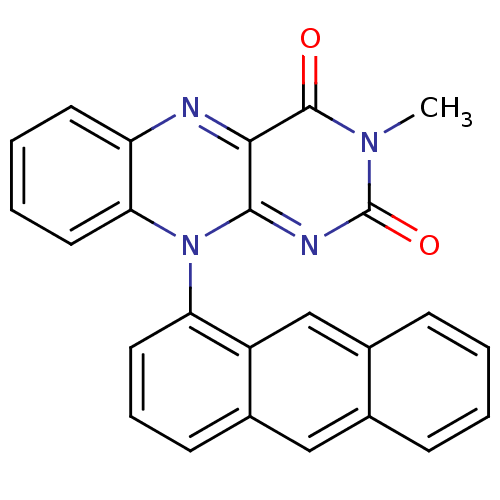
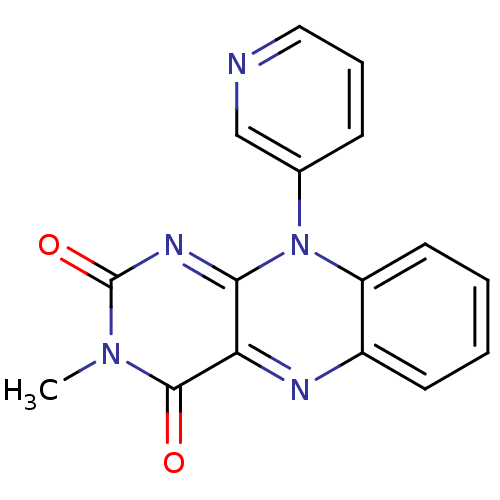
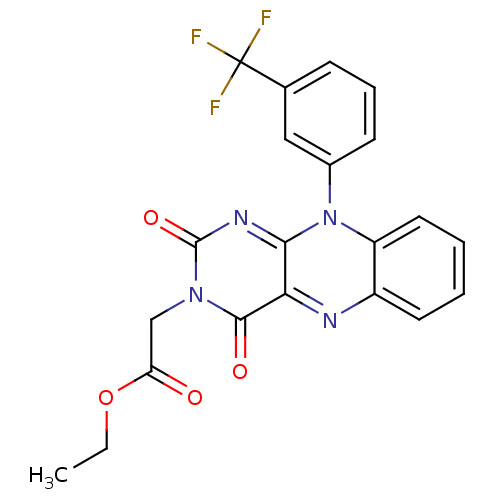
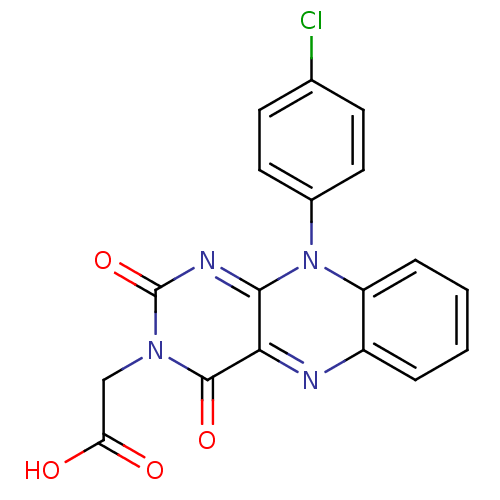
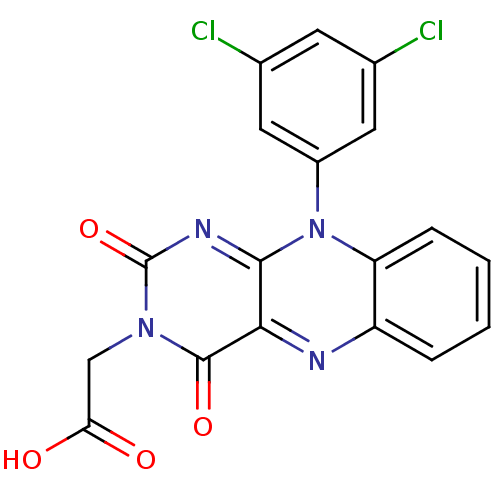
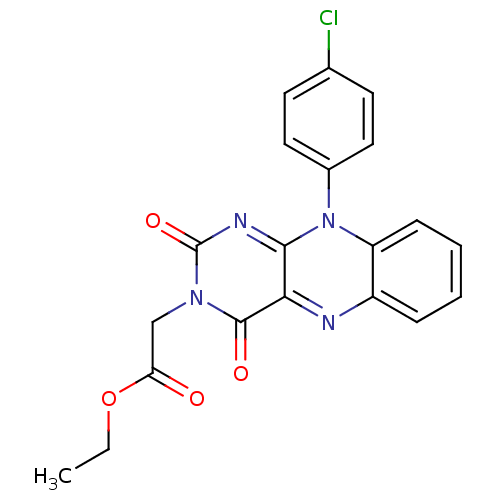
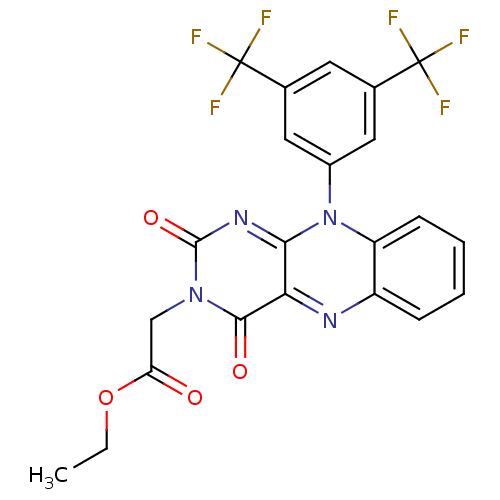
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair
TargetGlutathione reductase, mitochondrial(Homo sapiens (Human))
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Institut FüR Biochemie Ii Der UniversitäT
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory activity against human glutathione reductase in presence of 100 microM GSSGMore data for this Ligand-Target Pair