Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Ubiquitin carboxyl-terminal hydrolase 7
Ligand
BDBM507617
Substrate
n/a
Meas. Tech.
Evaluation of the Inhibition of USP7 by the Fluorescence Intensity (FLINT) Readings
IC50
61.5±n/a nM
Citation
 Kotschy, A; Wéber, C; Vasas, A; Molnár, B; Kiss, null; Macias, A; Murray, JB; Lewkowicz, E; Geneste, O; Chanrion, M; Demarles, D Substituted piperidines for the treatment of cancer US Patent US11046681 Publication Date 6/29/2021
Kotschy, A; Wéber, C; Vasas, A; Molnár, B; Kiss, null; Macias, A; Murray, JB; Lewkowicz, E; Geneste, O; Chanrion, M; Demarles, D Substituted piperidines for the treatment of cancer US Patent US11046681 Publication Date 6/29/2021 More Info.:
Target
Name:
Ubiquitin carboxyl-terminal hydrolase 7
Synonyms:
Deubiquitinating enzyme 7 | HAUSP | Herpesvirus-associated ubiquitin-specific protease | UBP7_HUMAN | USP7 | Ubiquitin thioesterase 7 | Ubiquitin-specific-processing protease 7
Type:
PROTEIN
Mol. Mass.:
128274.45
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1469483
Residue:
1102
Sequence:
MNHQQQQQQQKAGEQQLSEPEDMEMEAGDTDDPPRITQNPVINGNVALSDGHNTAEEDMEDDTSWRSEATFQFTVERFSRLSESVLSPPCFVRNLPWKIMVMPRFYPDRPHQKSVGFFLQCNAESDSTSWSCHAQAVLKIINYRDDEKSFSRRISHLFFHKENDWGFSNFMAWSEVTDPEKGFIDDDKVTFEVFVQADAPHGVAWDSKKHTGYVGLKNQGATCYMNSLLQTLFFTNQLRKAVYMMPTEGDDSSKSVPLALQRVFYELQHSDKPVGTKKLTKSFGWETLDSFMQHDVQELCRVLLDNVENKMKGTCVEGTIPKLFRGKMVSYIQCKEVDYRSDRREDYYDIQLSIKGKKNIFESFVDYVAVEQLDGDNKYDAGEHGLQEAEKGVKFLTLPPVLHLQLMRFMYDPQTDQNIKINDRFEFPEQLPLDEFLQKTDPKDPANYILHAVLVHSGDNHGGHYVVYLNPKGDGKWCKFDDDVVSRCTKEEAIEHNYGGHDDDLSVRHCTNAYMLVYIRESKLSEVLQAVTDHDIPQQLVERLQEEKRIEAQKRKERQEAHLYMQVQIVAEDQFCGHQGNDMYDEEKVKYTVFKVLKNSSLAEFVQSLSQTMGFPQDQIRLWPMQARSNGTKRPAMLDNEADGNKTMIELSDNENPWTIFLETVDPELAASGATLPKFDKDHDVMLFLKMYDPKTRSLNYCGHIYTPISCKIRDLLPVMCDRAGFIQDTSLILYEEVKPNLTERIQDYDVSLDKALDELMDGDIIVFQKDDPENDNSELPTAKEYFRDLYHRVDVIFCDKTIPNDPGFVVTLSNRMNYFQVAKTVAQRLNTDPMLLQFFKSQGYRDGPGNPLRHNYEGTLRDLLQFFKPRQPKKLYYQQLKMKITDFENRRSFKCIWLNSQFREEEITLYPDKHGCVRDLLEECKKAVELGEKASGKLRLLEIVSYKIIGVHQEDELLECLSPATSRTFRIEEIPLDQVDIDKENEMLVTVAHFHKEVFGTFGIPFLLRIHQGEHFREVMKRIQSLLDIQEKEFEKFKFAIVMMGRHQYINEDEYEVNLKDFEPQPGNMSHPRPWLGLDHFNKAPKRSRYTYLEKAIKIHN
Inhibitor
Name:
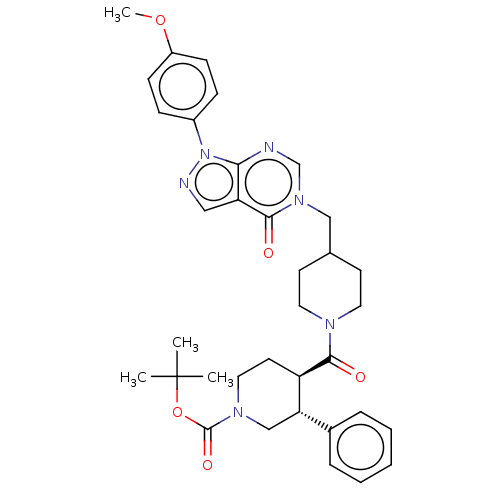
BDBM507617
Synonyms:
US11046681, Example 44 | tert-butyl (3R,4R)-4-[4-hydroxy-4-[[1-(4-methoxyphenyl)-4-oxo-pyrazolo[3,4-d]pyrimidin-5-yl]methyl]piperidine-1-carbonyl]-3-phenyl-piperidine-1-carboxylate
Type:
Small organic molecule
Emp. Form.:
C35H42N6O5
Mol. Mass.:
626.7452
SMILES:
COc1ccc(cc1)-n1ncc2c1ncn(CC1CCN(CC1)C(=O)[C@@H]1CCN(C[C@H]1c1ccccc1)C(=O)OC(C)(C)C)c2=O |r|