Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Acetylcholinesterase
Ligand
BDBM10613
Substrate
BDBM8959
Meas. Tech.
Cholinesterase Inhibition Assay
pH
8±n/a
Temperature
310.15±n/a K
IC50
760±20 nM
Citation
 Luo, W; Yu, QS; Kulkarni, SS; Parrish, DA; Holloway, HW; Tweedie, D; Shafferman, A; Lahiri, DK; Brossi, A; Greig, NH Inhibition of human acetyl- and butyrylcholinesterase by novel carbamates of (-)- and (+)-tetrahydrofurobenzofuran and methanobenzodioxepine. J Med Chem 49:2174-85 (2006) [PubMed] Article
Luo, W; Yu, QS; Kulkarni, SS; Parrish, DA; Holloway, HW; Tweedie, D; Shafferman, A; Lahiri, DK; Brossi, A; Greig, NH Inhibition of human acetyl- and butyrylcholinesterase by novel carbamates of (-)- and (+)-tetrahydrofurobenzofuran and methanobenzodioxepine. J Med Chem 49:2174-85 (2006) [PubMed] Article More Info.:
Target
Name:
Acetylcholinesterase
Synonyms:
ACES_HUMAN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase (human AChE)
Type:
Enzyme
Mol. Mass.:
67792.70
Organism:
Homo sapiens (Human)
Description:
P22303
Residue:
614
Sequence:
MRPPQCLLHTPSLASPLLLLLLWLLGGGVGAEGREDAELLVTVRGGRLRGIRLKTPGGPVSAFLGIPFAEPPMGPRRFLPPEPKQPWSGVVDATTFQSVCYQYVDTLYPGFEGTEMWNPNRELSEDCLYLNVWTPYPRPTSPTPVLVWIYGGGFYSGASSLDVYDGRFLVQAERTVLVSMNYRVGAFGFLALPGSREAPGNVGLLDQRLALQWVQENVAAFGGDPTSVTLFGESAGAASVGMHLLSPPSRGLFHRAVLQSGAPNGPWATVGMGEARRRATQLAHLVGCPPGGTGGNDTELVACLRTRPAQVLVNHEWHVLPQESVFRFSFVPVVDGDFLSDTPEALINAGDFHGLQVLVGVVKDEGSYFLVYGAPGFSKDNESLISRAEFLAGVRVGVPQVSDLAAEAVVLHYTDWLHPEDPARLREALSDVVGDHNVVCPVAQLAGRLAAQGARVYAYVFEHRASTLSWPLWMGVPHGYEIEFIFGIPLDPSRNYTAEEKIFAQRLMRYWANFARTGDPNEPRDPKAPQWPPYTAGAQQYVSLDLRPLEVRRGLRAQACAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKNQFDHYSKQDRCSDL
Inhibitor
Name:
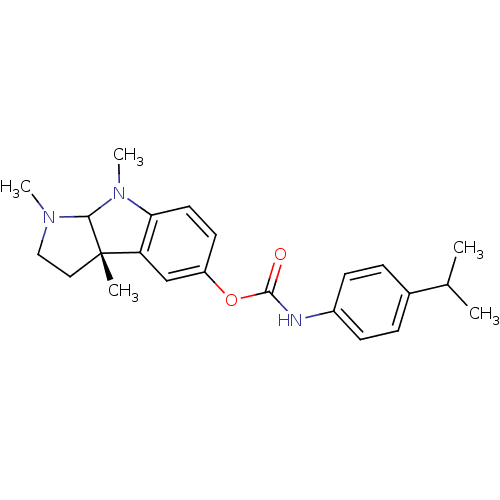
BDBM10613
Synonyms:
(3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b]indol-5-yl N-[4-(propan-2-yl)phenyl]carbamate | CHEMBL417915 | Physostigmine Carbamate 18a
Type:
Small organic molecule
Emp. Form.:
C23H29N3O2
Mol. Mass.:
379.4953
SMILES:
CC(C)c1ccc(NC(=O)Oc2ccc3N(C)C4N(C)CC[C@@]4(C)c3c2)cc1 |r|
Substrate
Name:
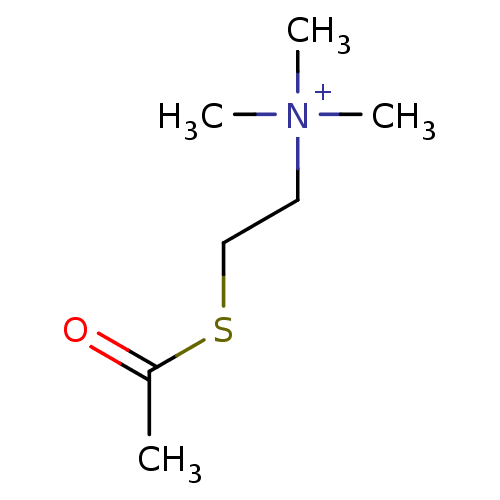
BDBM8959
Synonyms:
(2-Mercaptoethyl)trimethylammonium iodide acetate | ATC | Acetylthiocholine | [2-(acetylsulfanyl)ethyl]trimethylazanium iodide | acetylthiocholine chloride | acetylthiocholine iodide
Type:
Small organic molecule
Emp. Form.:
C7H16NOS
Mol. Mass.:
162.272
SMILES:
CC(=O)SCC[N+](C)(C)C