Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Acetylcholinesterase
Ligand
BDBM10985
Substrate
BDBM8959
Meas. Tech.
Cholinesterase Inhibition Assay
IC50
690±n/a nM
Citation
 Yu, Q; Holloway, HW; Flippen-Anderson, JL; Hoffman, B; Brossi, A; Greig, NH Methyl analogues of the experimental Alzheimer drug phenserine: synthesis and structure/activity relationships for acetyl- and butyrylcholinesterase inhibitory action. J Med Chem 44:4062-71 (2001) [PubMed] Article
Yu, Q; Holloway, HW; Flippen-Anderson, JL; Hoffman, B; Brossi, A; Greig, NH Methyl analogues of the experimental Alzheimer drug phenserine: synthesis and structure/activity relationships for acetyl- and butyrylcholinesterase inhibitory action. J Med Chem 44:4062-71 (2001) [PubMed] Article More Info.:
Target
Name:
Acetylcholinesterase
Synonyms:
ACES_HUMAN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase (human AChE)
Type:
Enzyme
Mol. Mass.:
67792.70
Organism:
Homo sapiens (Human)
Description:
P22303
Residue:
614
Sequence:
MRPPQCLLHTPSLASPLLLLLLWLLGGGVGAEGREDAELLVTVRGGRLRGIRLKTPGGPVSAFLGIPFAEPPMGPRRFLPPEPKQPWSGVVDATTFQSVCYQYVDTLYPGFEGTEMWNPNRELSEDCLYLNVWTPYPRPTSPTPVLVWIYGGGFYSGASSLDVYDGRFLVQAERTVLVSMNYRVGAFGFLALPGSREAPGNVGLLDQRLALQWVQENVAAFGGDPTSVTLFGESAGAASVGMHLLSPPSRGLFHRAVLQSGAPNGPWATVGMGEARRRATQLAHLVGCPPGGTGGNDTELVACLRTRPAQVLVNHEWHVLPQESVFRFSFVPVVDGDFLSDTPEALINAGDFHGLQVLVGVVKDEGSYFLVYGAPGFSKDNESLISRAEFLAGVRVGVPQVSDLAAEAVVLHYTDWLHPEDPARLREALSDVVGDHNVVCPVAQLAGRLAAQGARVYAYVFEHRASTLSWPLWMGVPHGYEIEFIFGIPLDPSRNYTAEEKIFAQRLMRYWANFARTGDPNEPRDPKAPQWPPYTAGAQQYVSLDLRPLEVRRGLRAQACAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKNQFDHYSKQDRCSDL
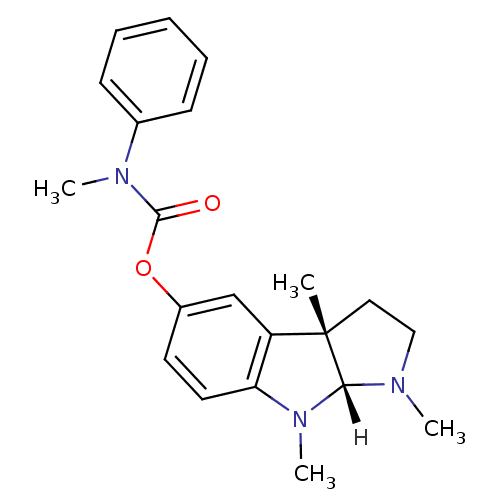
Inhibitor
Name:
BDBM10985
Synonyms:
(3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indol-5-yl methyl(phenyl)carbamate | (3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b]indol-5-yl N-methyl-N-phenylcarbamate | N-methylphenserine | Phenserine analog 12
Type:
Small organic molecule
Emp. Form.:
C21H25N3O2
Mol. Mass.:
351.4421
SMILES:
[H][C@]12N(C)CC[C@@]1(C)c1cc(OC(=O)N(C)c3ccccc3)ccc1N2C |r|
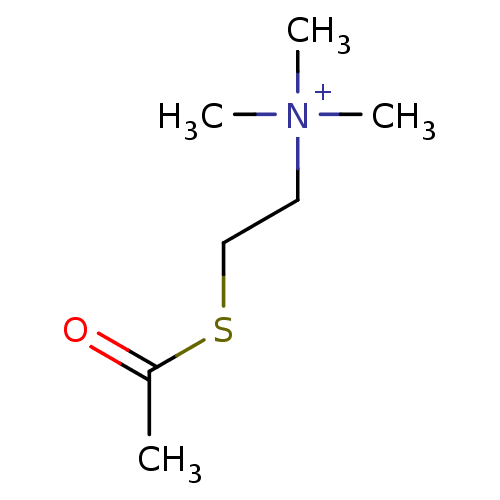
Substrate
Name:
BDBM8959
Synonyms:
(2-Mercaptoethyl)trimethylammonium iodide acetate | ATC | Acetylthiocholine | [2-(acetylsulfanyl)ethyl]trimethylazanium iodide | acetylthiocholine chloride | acetylthiocholine iodide
Type:
Small organic molecule
Emp. Form.:
C7H16NOS
Mol. Mass.:
162.272
SMILES:
CC(=O)SCC[N+](C)(C)C