Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Serine/threonine-protein kinase B-raf [V600E]
Ligand
BDBM333665
Substrate
n/a
Meas. Tech.
Competition Binding Assay
Kd
<250±n/a nM
Citation
 Abraham, S; Bhagwat, SS; Campbell, BT; Chao, Q; Faraoni, R; Holladay, MW; Lai, AG; Rowbottom, MW; Setti, E; Sprankle, KG RAF kinase modulator compounds and methods of use thereof US Patent US9730937 Publication Date 8/15/2017
Abraham, S; Bhagwat, SS; Campbell, BT; Chao, Q; Faraoni, R; Holladay, MW; Lai, AG; Rowbottom, MW; Setti, E; Sprankle, KG RAF kinase modulator compounds and methods of use thereof US Patent US9730937 Publication Date 8/15/2017 More Info.:
Target
Name:
Serine/threonine-protein kinase B-raf [V600E]
Synonyms:
B-RAF V600E | B-Raf (V600E) | B-Raf Protein Kinase Mutant (V600E) | B-Raf proto-oncogene serine/threonine-protein kinase | BRAF | BRAF (V600E) | BRAF mutant V600E | BRAF1 | BRAF_HUMAN | P15056 | Protein mono-ADP-ribosyltransferase (PARP3) | RAF serine/threonine protein kinase (V600E) | RAFB1 | Serine/threonine-protein kinase B-raf (V600E) | Serine/threonine-protein kinase B-raf (V600E) | Serine/threonine-protein kinase B-raf [V600E] | V-RAF murine sarcoma viral oncogene homologue B1 mutant (BRAF V600E)
Type:
n/a
Mol. Mass.:
84474.98
Organism:
Homo sapiens (Human)
Description:
P15056 V600E
Residue:
766
Sequence:
MAALSGGGGGGAEPGQALFNGDMEPEAGAGAGAAASSAADPAIPEEVWNIKQMIKLTQEHIEALLDKFGGEHNPPSIYLEAYEEYTSKLDALQQREQQLLESLGNGTDFSVSSSASMDTVTSSSSSSLSVLPSSLSVFQNPTDVARSNPKSPQKPIVRVFLPNKQRTVVPARCGVTVRDSLKKALMMRGLIPECCAVYRIQDGEKKPIGWDTDISWLTGEELHVEVLENVPLTTHNFVRKTFFTLAFCDFCRKLLFQGFRCQTCGYKFHQRCSTEVPLMCVNYDQLDLLFVSKFFEHHPIPQEEASLAETALTSGSSPSAPASDSIGPQILTSPSPSKSIPIPQPFRPADEDHRNQFGQRDRSSSAPNVHINTIEPVNIDDLIRDQGFRGDGGSTTGLSATPPASLPGSLTNVKALQKSPGPQRERKSSSSSEDRNRMKTLGRRDSSDDWEIPDGQITVGQRIGSGSFGTVYKGKWHGDVAVKMLNVTAPTPQQLQAFKNEVGVLRKTRHVNILLFMGYSTKPQLAIVTQWCEGSSLYHHLHIIETKFEMIKLIDIARQTAQGMDYLHAKSIIHRDLKSNNIFLHEDLTVKIGDFGLATEKSRWSGSHQFEQLSGSILWMAPEVIRMQDKNPYSFQSDVYAFGIVLYELMTGQLPYSNINNRDQIIFMVGRGYLSPDLSKVRSNCPKAMKRLMAECLKKKRDERPLFPQILASIELLARSLPKIHRSASEPSLNRAGFQTEDFSLYACASPKTPIQAGGYGAFPVH
Inhibitor
Name:
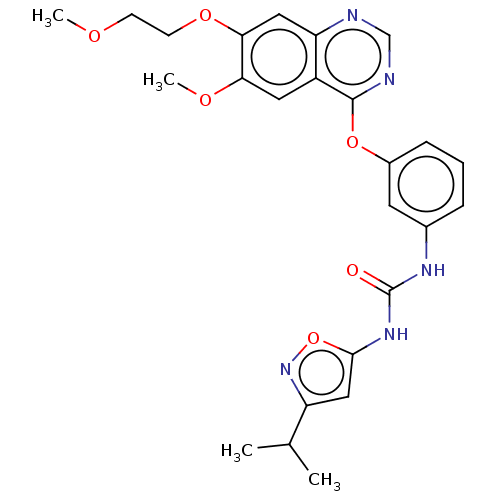
BDBM333665
Synonyms:
1-(3- isopropylisoxazol- 5-yl)- 3-(3-(6-methoxy- 7-(2- methoxyethoxy) quinazolin-4- yloxy)phenyl) urea | US9730937, Example 215
Type:
Small organic molecule
Emp. Form.:
C25H27N5O6
Mol. Mass.:
493.5118
SMILES:
COCCOc1cc2ncnc(Oc3cccc(NC(=O)Nc4cc(no4)C(C)C)c3)c2cc1OC