Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Coagulation factor VII
Ligand
BDBM13587
Substrate
BDBM13553
Meas. Tech.
Serine Protease Inhibition Assay
pH
7.8±n/a
Temperature
295.15±n/a K
Ki
200±n/a nM
Citation
 Groebke Zbinden, K; Banner, DW; Hilpert, K; Himber, J; Lave, T; Riederer, MA; Stahl, M; Tschopp, TB; Obst-Sander, U Dose-dependent antithrombotic activity of an orally active tissue factor/factor VIIa inhibitor without concomitant enhancement of bleeding propensity. Bioorg Med Chem 14:5357-69 (2006) [PubMed] Article
Groebke Zbinden, K; Banner, DW; Hilpert, K; Himber, J; Lave, T; Riederer, MA; Stahl, M; Tschopp, TB; Obst-Sander, U Dose-dependent antithrombotic activity of an orally active tissue factor/factor VIIa inhibitor without concomitant enhancement of bleeding propensity. Bioorg Med Chem 14:5357-69 (2006) [PubMed] Article More Info.:
Target
Name:
Coagulation factor VII
Synonyms:
Eptacog alfa | F7 | FA7_HUMAN | Factor VIIa | Factor VIIa (fVIIa) | Proconvertin | SPCA | Thrombin and coagulation factor VII | serum prothrombin conversion accelerator
Type:
Enzyme
Mol. Mass.:
51599.89
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
466
Sequence:
MVSQALRLLCLLLGLQGCLAAGGVAKASGGETRDMPWKPGPHRVFVTQEEAHGVLHRRRRANAFLEELRPGSLERECKEEQCSFEEAREIFKDAERTKLFWISYSDGDQCASSPCQNGGSCKDQLQSYICFCLPAFEGRNCETHKDDQLICVNENGGCEQYCSDHTGTKRSCRCHEGYSLLADGVSCTPTVEYPCGKIPILEKRNASKPQGRIVGGKVCPKGECPWQVLLLVNGAQLCGGTLINTIWVVSAAHCFDKIKNWRNLIAVLGEHDLSEHDGDEQSRRVAQVIIPSTYVPGTTNHDIALLRLHQPVVLTDHVVPLCLPERTFSERTLAFVRFSLVSGWGQLLDRGATALELMVLNVPRLMTQDCLQQSRKVGDSPNITEYMFCAGYSDGSKDSCKGDSGGPHATHYRGTWYLTGIVSWGQGCATVGHFGVYTRVSQYIEWLQKLMRSEPRPGVLLRAPFP
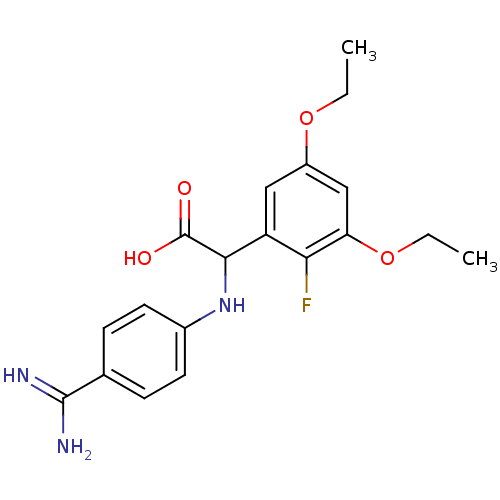
Inhibitor
Name:
BDBM13587
Synonyms:
(RS)-(4-carbamimidoyl-phenylamino)-(3,5-diethoxy-2-fluoro-phenyl)-acetic acid | 2-[(4-carbamimidoylphenyl)amino]-2-(3,5-diethoxy-2-fluorophenyl)acetic acid | phenylglycine derivative 2
Type:
Small organic molecule
Emp. Form.:
C19H22FN3O4
Mol. Mass.:
375.3941
SMILES:
CCOc1cc(OCC)c(F)c(c1)C(Nc1ccc(cc1)C(N)=N)C(O)=O
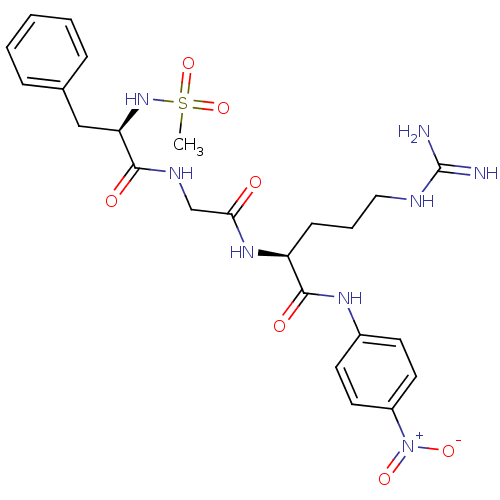
Substrate
Name:
BDBM13553
Synonyms:
(2S)-5-carbamimidamido-2-{2-[(2R)-2-methanesulfonamido-3-phenylpropanamido]acetamido}-N-(4-nitrophenyl)pentanamide; acetic acid | Chromozym tPA | N-Methylsulfonyl-D-Phe-Gly-Arg-4-nitranilide acetate
Type:
Small organic molecule
Emp. Form.:
C24H32N8O7S
Mol. Mass.:
576.625
SMILES:
CS(=O)(=O)N[C@H](Cc1ccccc1)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)Nc1ccc(cc1)[N+]([O-])=O |r|