Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prothrombin
Ligand
BDBM13590
Substrate
BDBM13573
Meas. Tech.
Serine Protease Inhibition Assay
pH
7.8±n/a
Temperature
295.15±n/a K
Ki
4500±n/a nM
Citation
 Groebke Zbinden, K; Banner, DW; Hilpert, K; Himber, J; Lave, T; Riederer, MA; Stahl, M; Tschopp, TB; Obst-Sander, U Dose-dependent antithrombotic activity of an orally active tissue factor/factor VIIa inhibitor without concomitant enhancement of bleeding propensity. Bioorg Med Chem 14:5357-69 (2006) [PubMed] Article
Groebke Zbinden, K; Banner, DW; Hilpert, K; Himber, J; Lave, T; Riederer, MA; Stahl, M; Tschopp, TB; Obst-Sander, U Dose-dependent antithrombotic activity of an orally active tissue factor/factor VIIa inhibitor without concomitant enhancement of bleeding propensity. Bioorg Med Chem 14:5357-69 (2006) [PubMed] Article More Info.:
Target
Name:
Prothrombin
Synonyms:
Activation peptide fragment 1 | Activation peptide fragment 2 | Coagulation factor II | F2 | Prothrombin precursor | THRB_HUMAN | Thrombin heavy chain | Thrombin light chain
Type:
Protein
Mol. Mass.:
70029.57
Organism:
Homo sapiens (Human)
Description:
P00734
Residue:
622
Sequence:
MAHVRGLQLPGCLALAALCSLVHSQHVFLAPQQARSLLQRVRRANTFLEEVRKGNLERECVEETCSYEEAFEALESSTATDVFWAKYTACETARTPRDKLAACLEGNCAEGLGTNYRGHVNITRSGIECQLWRSRYPHKPEINSTTHPGADLQENFCRNPDSSTTGPWCYTTDPTVRRQECSIPVCGQDQVTVAMTPRSEGSSVNLSPPLEQCVPDRGQQYQGRLAVTTHGLPCLAWASAQAKALSKHQDFNSAVQLVENFCRNPDGDEEGVWCYVAGKPGDFGYCDLNYCEEAVEEETGDGLDEDSDRAIEGRTATSEYQTFFNPRTFGSGEADCGLRPLFEKKSLEDKTERELLESYIDGRIVEGSDAEIGMSPWQVMLFRKSPQELLCGASLISDRWVLTAAHCLLYPPWDKNFTENDLLVRIGKHSRTRYERNIEKISMLEKIYIHPRYNWRENLDRDIALMKLKKPVAFSDYIHPVCLPDRETAASLLQAGYKGRVTGWGNLKETWTANVGKGQPSVLQVVNLPIVERPVCKDSTRIRITDNMFCAGYKPDEGKRGDACEGDSGGPFVMKSPFNNRWYQMGIVSWGEGCDRDGKYGFYTHVFRLKKWIQKVIDQFGE
Inhibitor
Name:
BDBM13590
Synonyms:
(2R)-2-[(4-carbamimidoylphenyl)amino]-2-{5-ethoxy-2-fluoro-3-[(3R)-oxolan-3-yloxy]phenyl}acetic acid | (R)-(4-Carbamimidoyl-phenylamino)-{3-ethoxy-2-fluoro-5-[(R)-(tetra-hydro-furan-3-yl)-oxy]-phenyl}-acetic acid | phenylglycine derivative 5
Type:
Small organic molecule
Emp. Form.:
C21H24FN3O5
Mol. Mass.:
417.4308
SMILES:
CCOc1cc(O[C@@H]2CCOC2)c(F)c(c1)[C@@H](Nc1ccc(cc1)C(N)=N)C(O)=O |r|
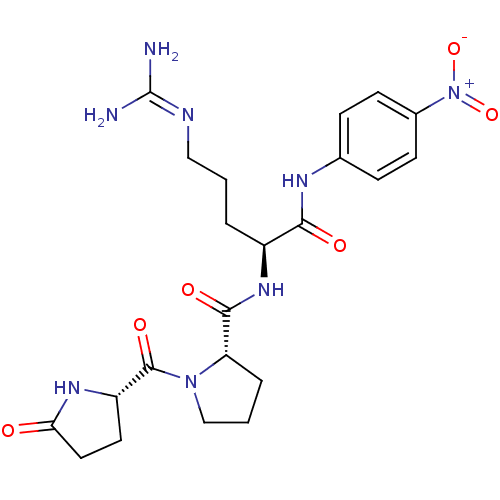
Substrate
Name:
BDBM13573
Synonyms:
(2S)-5-carbamimidamido-N-(4-nitrophenyl)-2-{[(2S)-1-{[(2S)-5-oxopyrrolidin-2-yl]carbonyl}pyrrolidin-2-yl]formamido}pentanamide hydrochloride | Chromogenic Substrate S-2366 | Glu-Pro-Arg-pNA | Hepsin Chromogenic Substrate | L-Pyroglutamyl-L-prolyl-L-arginine-p-Nitroaniline | L-Pyroglutamyl-L-prolyl-L-argininep-Nitroaniline | S-2366
Type:
Small organic molecule
Emp. Form.:
C22H30N8O6
Mol. Mass.:
502.5236
SMILES:
[#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-c1ccc(cc1)-[#7+](-[#8-])=O |r|