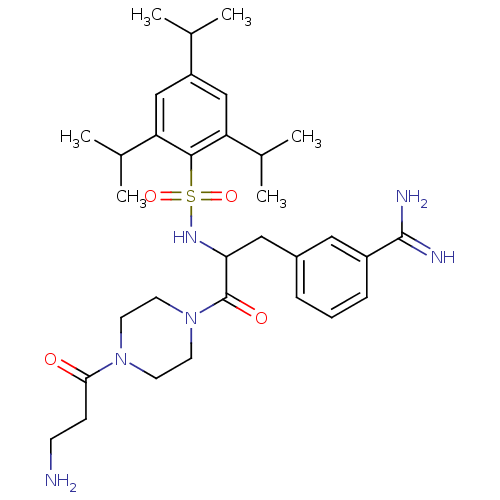
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Serine protease 1
Ligand
BDBM16174
Substrate
BDBM12774
Meas. Tech.
Determination of Inhibition Constants
pH
8±n/a
Temperature
298.15±n/a K
Ki
600±n/a nM
Citation
 Zeslawska, E; Schweinitz, A; Karcher, A; Sondermann, P; Sperl, S; Sturzebecher, J; Jacob, U Crystals of the urokinase type plasminogen activator variant beta(c)-uPAin complex with small molecule inhibitors open the way towards structure-based drug design. J Mol Biol 301:465-75 (2000) [PubMed] Article
Zeslawska, E; Schweinitz, A; Karcher, A; Sondermann, P; Sperl, S; Sturzebecher, J; Jacob, U Crystals of the urokinase type plasminogen activator variant beta(c)-uPAin complex with small molecule inhibitors open the way towards structure-based drug design. J Mol Biol 301:465-75 (2000) [PubMed] Article More Info.:
Target
Name:
Serine protease 1
Synonyms:
Beta-Trypsin | Cationic trypsin | PRSS1 | TRP1 | TRY1 | TRY1_BOVIN | TRYP1 | Trypsin | Trypsin I
Type:
Enzyme
Mol. Mass.:
25790.52
Organism:
Bos taurus (bovine)
Description:
P00760
Residue:
246
Sequence:
MKTFIFLALLGAAVAFPVDDDDKIVGGYTCGANTVPYQVSLNSGYHFCGGSLINSQWVVSAAHCYKSGIQVRLGEDNINVVEGNEQFISASKSIVHPSYNSNTLNNDIMLIKLKSAASLNSRVASISLPTSCASAGTQCLISGWGNTKSSGTSYPDVLKCLKAPILSDSSCKSAYPGQITSNMFCAGYLEGGKDSCQGDSGGPVVCSGKLQGIVSWGSGCAQKNKPGVYTKVCNYVSWIKQTIASN
Inhibitor
Name:
BDBM16174
Synonyms:
3-[3-(4-beta-alanylpiperazin-1-yl)-3-oxo-2-({[2,4,6-tris(1-methylethyl)phenyl]sulfonyl}amino)propyl]benzenecarboximidamide | 3-{3-[4-(3-aminopropanoyl)piperazin-1-yl]-3-oxo-2-{[2,4,6-tris(propan-2-yl)benzene]sulfonamido}propyl}benzene-1-carboximidamide | UKI-1D
Type:
Small organic molecule
Emp. Form.:
C32H48N6O4S
Mol. Mass.:
612.826
SMILES:
CC(C)c1cc(C(C)C)c(c(c1)C(C)C)S(=O)(=O)NC(Cc1cccc(c1)C(N)=N)C(=O)N1CCN(CC1)C(=O)CCN
Substrate
Name:
BDBM12774
Synonyms:
(2S)-5-carbamimidamido-2-{2-[(2R)-3-(4-hydroxycyclohexyl)-2-methanesulfonamidopropanamido]acetamido}-N-(4-nitrophenyl)pentanamide; acetic acid | CH3-SO2-D-HHT-Gly-Arg-pNA | Factor IX Chromogenic Substrate | MS-D-HHT-Gly-Arg-pNA | Methylsulfonyl-D-hexahydrotyrosyl-glycyl-arginine-4-nitroanilide | N-Methylsulfonyl-D-hexahydrotyrosyl-glycyl-L-arginine-4-nitroanilide acetate | Spectrozyme tPA | tPA/Trypsin Substrate
Type:
Small organic molecule
Emp. Form.:
C24H38N8O8S
Mol. Mass.:
598.672
SMILES:
CS(=O)(=O)NC(CC1CCC(O)CC1)C(=O)NCC(=O)NC(CCC\[NH+]=C(\N)[NH-])C(=O)Nc1ccc(cc1)[N+]([O-])=O |(-14.85,4.9,;-14.08,3.57,;-12.75,4.34,;-15.42,2.8,;-13.31,2.23,;-11.77,2.23,;-11,.9,;-11.77,-.43,;-13.31,-.43,;-14.08,-1.77,;-13.31,-3.1,;-14.08,-4.44,;-11.77,-3.1,;-11,-1.77,;-11,3.57,;-11.77,4.9,;-9.46,3.57,;-8.69,2.23,;-7.15,2.23,;-6.38,3.57,;-6.38,.9,;-4.84,.9,;-4.07,-.43,;-4.84,-1.77,;-4.07,-3.1,;-4.84,-4.44,;-4.07,-5.77,;-4.84,-7.1,;-2.53,-5.77,;-4.07,2.23,;-4.84,3.57,;-2.53,2.23,;-1.76,3.57,;-2.53,4.9,;-1.76,6.23,;-.22,6.23,;.55,4.9,;-.22,3.57,;.55,7.57,;-.22,8.9,;2.09,7.57,)|