Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Coagulation factor X
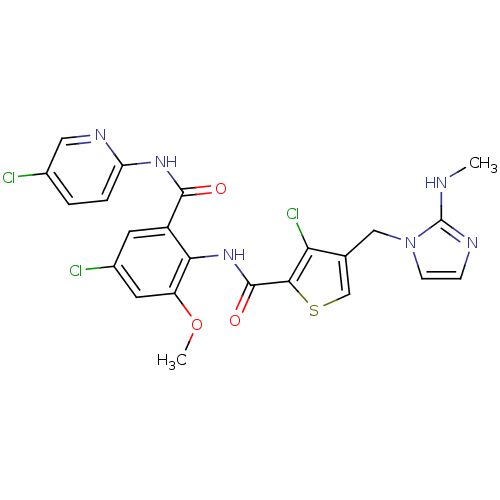
Ligand
BDBM17135
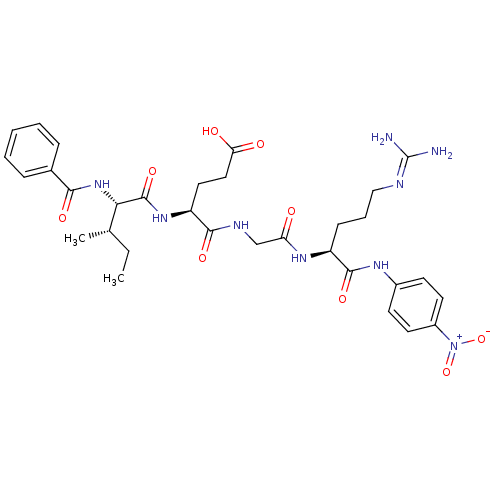
Substrate
BDBM12658
Meas. Tech.
Enzyme Inhibition Assay
Ki
0.31±n/a nM
Citation
 Ye, B; Arnaiz, DO; Chou, YL; Griedel, BD; Karanjawala, R; Lee, W; Morrissey, MM; Sacchi, KL; Sakata, ST; Shaw, KJ; Wu, SC; Zhao, Z; Adler, M; Cheeseman, S; Dole, WP; Ewing, J; Fitch, R; Lentz, D; Liang, A; Light, D; Morser, J; Post, J; Rumennik, G; Subramanyam, B; Sullivan, ME; Vergona, R; Walters, J; Wang, YX; White, KA; Whitlow, M; Kochanny, MJ Thiophene-anthranilamides as highly potent and orally available factor xa inhibitors J Med Chem 50:2967-80 (2007) [PubMed] Article
Ye, B; Arnaiz, DO; Chou, YL; Griedel, BD; Karanjawala, R; Lee, W; Morrissey, MM; Sacchi, KL; Sakata, ST; Shaw, KJ; Wu, SC; Zhao, Z; Adler, M; Cheeseman, S; Dole, WP; Ewing, J; Fitch, R; Lentz, D; Liang, A; Light, D; Morser, J; Post, J; Rumennik, G; Subramanyam, B; Sullivan, ME; Vergona, R; Walters, J; Wang, YX; White, KA; Whitlow, M; Kochanny, MJ Thiophene-anthranilamides as highly potent and orally available factor xa inhibitors J Med Chem 50:2967-80 (2007) [PubMed] Article More Info.:
Target
Name:
Coagulation factor X
Synonyms:
Coagulation factor X precursor | Factor Xa (fXa) | Stuart factor | Stuart-Prower factor
Type:
Enzyme
Mol. Mass.:
17844.67
Organism:
Canis lupus familiaris (Dog)
Description:
n/a
Residue:
159
Sequence:
ILSEYYILTAAHCLQQAKKFTVRVGERDTDKEEGNEVAHEVEMIIKHNKFVRETYDFDIAVIKLKTPITFRMNVAPACLPQKDWAESTLMTQKTGIVSGFGKTHEKGRPSTTLKMMEVPYVDRNTCKLSSSFSITQNMFCAGYDSKPEDGCQGDSGGPH
Inhibitor
Name:
BDBM17135
Synonyms:
3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carbamoyl]-6-methoxyphenyl}-4-{[2-(methylamino)-1H-imidazol-1-yl]methyl}thiophene-2-carboxamide | Thiophene-Anthranilamide, 17m | ZK 813039 | ZK813039
Type:
Small organic molecule
Emp. Form.:
C23H19Cl3N6O3S
Mol. Mass.:
565.859
SMILES:
CNc1nccn1Cc1csc(C(=O)Nc2c(OC)cc(Cl)cc2C(=O)Nc2ccc(Cl)cn2)c1Cl
Substrate
Name:
BDBM12658
Synonyms:
4-[({[(1S)-4-carbamimidamido-1-[(4-nitrophenyl)carbamoyl]butyl]carbamoyl}methyl)carbamoyl]-4-[(2S,3S)-3-methyl-2-(phenylformamido)pentanamido]butanoic acid hydrochloride | Bz-Ile-Glu-Gly-Arg-pNA | Chromogenic Substrate S-2222 | L-Argininamide, N-benzoyl-L-isoleucyl-L-alpha-glutamylglycyl-N-(4-nitrophenyl)-, monohydrochloride | benzoyl-Ile-Glu-Gly-Arg-p-nitroanilide
Type:
Small organic molecule
Emp. Form.:
C32H43N9O9
Mol. Mass.:
697.7387
SMILES:
[#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-c1ccccc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-c1ccc(cc1)-[#7+](-[#8-])=O |r|