Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Angiotensin-converting enzyme
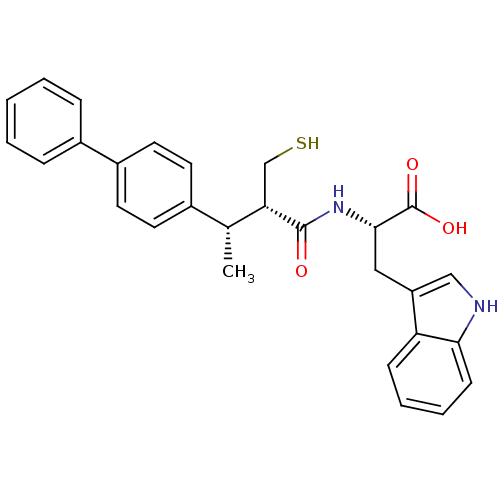
Ligand
BDBM21648
Substrate
BDBM21660
Meas. Tech.
In Vitro Inhibition of NEP
Ki
65±n/a nM
Citation
 Inguimbert, N; Coric, P; Poras, H; Meudal, H; Teffot, F; Fournié-Zaluski, MC; Roques, BP Toward an optimal joint recognition of the S1' subsites of endothelin converting enzyme-1 (ECE-1), angiotensin converting enzyme (ACE), and neutral endopeptidase (NEP). J Med Chem 45:1477-86 (2002) [PubMed] Article
Inguimbert, N; Coric, P; Poras, H; Meudal, H; Teffot, F; Fournié-Zaluski, MC; Roques, BP Toward an optimal joint recognition of the S1' subsites of endothelin converting enzyme-1 (ECE-1), angiotensin converting enzyme (ACE), and neutral endopeptidase (NEP). J Med Chem 45:1477-86 (2002) [PubMed] Article More Info.:
Target
Name:
Angiotensin-converting enzyme
Synonyms:
ACE_RAT | Ace | Angiotensin-converting enzyme | Dcp1
Type:
PROTEIN
Mol. Mass.:
150907.81
Organism:
Rattus norvegicus
Description:
ChEMBL_35219
Residue:
1313
Sequence:
MGAASGQRGRWPLSPPLLMLSLLLLLLLPPSPAPALDPGLQPGNFSADEAGAQLFADSYNSSAEVVMFQSTAASWAHDTNITEENARLQEEAALINQEFAEVWGKKAKELYESIWQNFTDQKLRRIIGSVQTLGPANLPLTQRLQYNSLLSNMSRIYSTGKVCFPNKTATCWSLDPELTNILASSRNYAKVLFAWEGWHDAVGIPLRPLYQDFTALSNEAYRQDGFSDTGAYWRSWYESPSFEESLEHLYHQVEPLYLNLHAFVRRALHRRYGDKYINLRGPIPAHLLGDMWAQSWENIYDMVVPFPDKPNLDVTSTMVQKGWNATHMFRVAEEFFTSLGLSPMPPEFWAESMLEKPADGREVVCHASAWDFYNRKDFRIKQCTRVTMDQLSTVHHEMGHVQYYLQYKDLHVSLRRGANPGFHEAIGDVLALSVSTPAHLHKIGLLDRVANDIESDINYLLKMALEKIAFLPFGYLVDQWRWGVFSGRTPPSRYNYDWWYLRTKYQGICPPVARNETHFDAGAKFHIPSVTPYIRYFVSFVLQFQFHQALCKEAGHQGPLHQCDIYQSTKAGAKLQQVLQAGCSRPWQEVLKDLVGSDALDASALMEYFQPVSQWLQEQNQRNGEVLGWPEYQWRPPLPDNYPEGIDLETDEAKANRFVEEYDRTAKVLWNEYAEANWHYNTNITIEGSKILLQKNKEVSNHTLKYGTWAKTFDVSNFQNSTIKRIIKKVQNVDRAVLPPNELEEYNQILLDMETTYSVANVCYTNGTCLSLEPDLTNIMATSRKYEELLWVWKSWRDKVGRAILPFFPKYVDFSNKIAKLNGYSDAGDSWRSSYESDDLEQDLEKLYQELQPLYLNLHAYVRRSLHRHYGSEYINLDGPIPAHLLGNMWAQTWSNIYDLVAPFPSAPSIDATEAMIKQGWTPRRIFKEADNFFTSLGLLPVPPEFWNKSMLEKPTDGREVVCHASAWDFYNGKDFRIKQCTSVNMEELVIAHHEMGHIQYFMQYKDLPVTFREGANPGFHEAIGDVLALSVSTPKHLHSLNLLSSEGSGYEHDINFLMKMALDKIAFIPFSYLIDQWRWRVFDGSITKENYNQEWWSLRLKYQGLCPPVPRSQGDFDPGSKFHVPANVPYIRYFISFIIQFQFHEALCRAAGHTGPLYKCDIYQSKEAGKLLADAMKLGYSKQWPEAMKIITGQPNMSASAIMNYFKPLTEWLVTENRRHGETLGWPEYTWTPNTARAEGSLPESSRVNFLGMYLEPQQARVGQWVLLFLGVALLVATVGLAHRLYNIHNHHSLRRPHRGPQFGSEVELRHS
Inhibitor
Name:
BDBM21648
Synonyms:
(2S)-3-(1H-indol-3-yl)-2-[(2S,3S)-3-(4-phenylphenyl)-2-(sulfanylmethyl)butanamido]propanoic acid | Mercaptoacyl amino acid compound, 10a
Type:
Small organic molecule
Emp. Form.:
C28H28N2O3S
Mol. Mass.:
472.599
SMILES:
C[C@@H]([C@H](CS)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O)c1ccc(cc1)-c1ccccc1 |r|