Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Poly(ADP-ribose) glycohydrolase
Ligand
BDBM371485
Substrate
n/a
Meas. Tech.
PARG Assay
IC50
25.0±n/a nM
Citation
 McGonagle, AE; Jordan, A; Waszkowycz, B; Hutton, C; Waddell, I; Hitchin, JR; Smith, KM; Hamilton, NM 2,4-dioxo-quinazoline-6-sulfonamide derivatives as inhibitors of PARG US Patent US10239843 Publication Date 3/26/2019
McGonagle, AE; Jordan, A; Waszkowycz, B; Hutton, C; Waddell, I; Hitchin, JR; Smith, KM; Hamilton, NM 2,4-dioxo-quinazoline-6-sulfonamide derivatives as inhibitors of PARG US Patent US10239843 Publication Date 3/26/2019 More Info.:
Target
Name:
Poly(ADP-ribose) glycohydrolase
Synonyms:
PARG | PARG_HUMAN | Poly(ADP-ribose) glycohydrolase | poly(ADP-ribose) glycohydrolase (PARG)
Type:
Protein
Mol. Mass.:
111107.13
Organism:
Homo sapiens (Human)
Description:
Q86W56
Residue:
976
Sequence:
MNAGPGCEPCTKRPRWGAATTSPAASDARSFPSRQRRVLDPKDAHVQFRVPPSSPACVPGRAGQHRGSATSLVFKQKTITSWMDTKGIKTAESESLDSKENNNTRIESMMSSVQKDNFYQHNVEKLENVSQLSLDKSPTEKSTQYLNQHQTAAMCKWQNEGKHTEQLLESEPQTVTLVPEQFSNANIDRSPQNDDHSDTDSEENRDNQQFLTTVKLANAKQTTEDEQAREAKSHQKCSKSCDPGEDCASCQQDEIDVVPESPLSDVGSEDVGTGPKNDNKLTRQESCLGNSPPFEKESEPESPMDVDNSKNSCQDSEADEETSPGFDEQEDGSSSQTANKPSRFQARDADIEFRKRYSTKGGEVRLHFQFEGGESRTGMNDLNAKLPGNISSLNVECRNSKQHGKKDSKITDHFMRLPKAEDRRKEQWETKHQRTERKIPKYVPPHLSPDKKWLGTPIEEMRRMPRCGIRLPLLRPSANHTVTIRVDLLRAGEVPKPFPTHYKDLWDNKHVKMPCSEQNLYPVEDENGERTAGSRWELIQTALLNKFTRPQNLKDAILKYNVAYSKKWDFTALIDFWDKVLEEAEAQHLYQSILPDMVKIALCLPNICTQPIPLLKQKMNHSITMSQEQIASLLANAFFCTFPRRNAKMKSEYSSYPDINFNRLFEGRSSRKPEKLKTLFCYFRRVTEKKPTGLVTFTRQSLEDFPEWERCEKPLTRLHVTYEGTIEENGQGMLQVDFANRFVGGGVTSAGLVQEEIRFLINPELIISRLFTEVLDHNECLIITGTEQYSEYTGYAETYRWSRSHEDGSERDDWQRRCTEIVAIDALHFRRYLDQFVPEKMRRELNKAYCGFLRPGVSSENLSAVATGNWGCGAFGGDARLKALIQILAAAAAERDVVYFTFGDSELMRDIYSMHIFLTERKLTVGDVYKLLLRYYNEECRNCSTPGPDIKLYPFIYHAVESCAETADHSGQRTGT
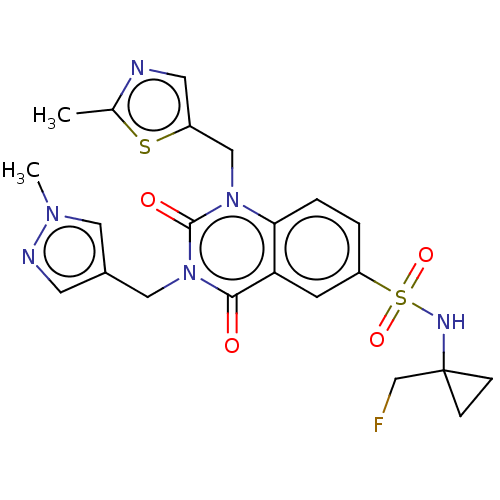
Inhibitor
Name:
BDBM371485
Synonyms:
N-[1-(Fluoromethyl)cyclopropyl]-3-[(1-methylpyrazol-4-yl)methyl]-1-[(2-methylthiazol-5-yl)methyl]-2,4-dioxo-quinazoline-6-sulfonamide | US10239843, Example 594
Type:
Small organic molecule
Emp. Form.:
C22H23FN6O4S2
Mol. Mass.:
518.584
SMILES:
Cc1ncc(Cn2c3ccc(cc3c(=O)n(Cc3cnn(C)c3)c2=O)S(=O)(=O)NC2(CF)CC2)s1