Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Indoleamine 2,3-dioxygenase 1
Ligand
BDBM21979
Substrate
BDBM21974
Meas. Tech.
Enzyme Inhibition Assay
pH
6.5±n/a
Temperature
310.15±n/a K
Ki
190±20 nM
Citation
More Info.:
Target
Name:
Indoleamine 2,3-dioxygenase 1
Synonyms:
I23O1_HUMAN | IDO | IDO-1 | IDO1 | INDO | Indoleamine 2,3-Dioxygenasae (IDO) | Indoleamine 2,3-dioxygenase | Indoleamine-pyrrole 2,3-dioxygenase
Type:
Enzyme
Mol. Mass.:
45330.80
Organism:
Homo sapiens (Human)
Description:
P14902
Residue:
403
Sequence:
MAHAMENSWTISKEYHIDEEVGFALPNPQENLPDFYNDWMFIAKHLPDLIESGQLRERVEKLNMLSIDHLTDHKSQRLARLVLGCITMAYVWGKGHGDVRKVLPRNIAVPYCQLSKKLELPPILVYADCVLANWKKKDPNKPLTYENMDVLFSFRDGDCSKGFFLVSLLVEIAAASAIKVIPTVFKAMQMQERDTLLKALLEIASCLEKALQVFHQIHDHVNPKAFFSVLRIYLSGWKGNPQLSDGLVYEGFWEDPKEFAGGSAGQSSVFQCFDVLLGIQQTAGGGHAAQFLQDMRRYMPPAHRNFLCSLESNPSVREFVLSKGDAGLREAYDACVKALVSLRSYHLQIVTKYILIPASQQPKENKTSEDPSKLEAKGTGGTDLMNFLKTVRSTTEKSLLKEG
Inhibitor
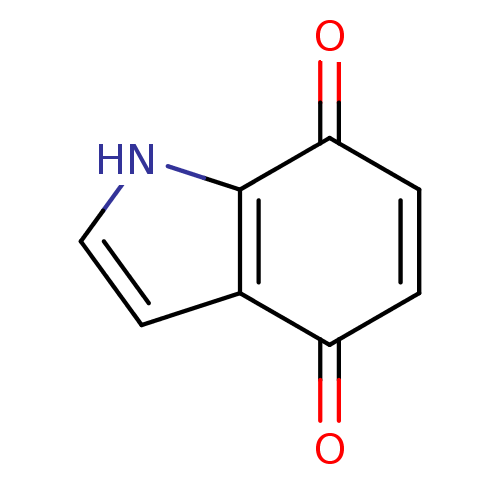
Name:
BDBM21979
Synonyms:
4,7-dihydro-1H-indole-4,7-dione | Indolequinone, 19
Type:
Small organic molecule
Emp. Form.:
C8H5NO2
Mol. Mass.:
147.1308
SMILES:
O=C1C=CC(=O)c2[nH]ccc12 |c:2|