Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prostaglandin G/H synthase 2
Ligand
BDBM22569
Substrate
BDBM22319
Meas. Tech.
Time-Dependent Inhibition Assay
pH
8±n/a
Temperature
310.15±n/a K
IC50
170±n/a nM
Citation
 Harman, CA; Turman, MV; Kozak, KR; Marnett, LJ; Smith, WL; Garavito, RM Structural basis of enantioselective inhibition of cyclooxygenase-1 by S-alpha-substituted indomethacin ethanolamides. J Biol Chem 282:28096-105 (2007) [PubMed] Article
Harman, CA; Turman, MV; Kozak, KR; Marnett, LJ; Smith, WL; Garavito, RM Structural basis of enantioselective inhibition of cyclooxygenase-1 by S-alpha-substituted indomethacin ethanolamides. J Biol Chem 282:28096-105 (2007) [PubMed] Article More Info.:
Target
Name:
Prostaglandin G/H synthase 2
Synonyms:
COX2 | Cyclooxygenase | Cyclooxygenase 2 (COX-2) | Cyclooxygenase-2 | Cyclooxygenase-2 (COX-2 AA) | Cyclooxygenase-2 (COX-2 AEA) | Cyclooxygenase-2 (COX-2) | PGH synthase 2 | PGH2_HUMAN | PGHS-2 | PHS II | PTGS2 | Prostaglandin E synthase/G/H synthase 2 | Prostaglandin H2 synthase 2 | Prostaglandin-endoperoxide synthase 2
Type:
Enzyme
Mol. Mass.:
69003.89
Organism:
Homo sapiens (Human)
Description:
Recombinant Cox-2 provided by Cayman (Cayman Chemical Co.,Ann Arbor, MI).
Residue:
604
Sequence:
MLARALLLCAVLALSHTANPCCSHPCQNRGVCMSVGFDQYKCDCTRTGFYGENCSTPEFLTRIKLFLKPTPNTVHYILTHFKGFWNVVNNIPFLRNAIMSYVLTSRSHLIDSPPTYNADYGYKSWEAFSNLSYYTRALPPVPDDCPTPLGVKGKKQLPDSNEIVEKLLLRRKFIPDPQGSNMMFAFFAQHFTHQFFKTDHKRGPAFTNGLGHGVDLNHIYGETLARQRKLRLFKDGKMKYQIIDGEMYPPTVKDTQAEMIYPPQVPEHLRFAVGQEVFGLVPGLMMYATIWLREHNRVCDVLKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNKQFQYQNRIAAEFNTLYHWHPLLPDTFQIHDQKYNYQQFIYNNSILLEHGITQFVESFTRQIAGRVAGGRNVPPAVQKVSQASIDQSRQMKYQSFNEYRKRFMLKPYESFEELTGEKEMSAELEALYGDIDAVELYPALLVEKPRPDAIFGETMVEVGAPFSLKGLMGNVICSPAYWKPSTFGGEVGFQIINTASIQSLICNNVKGCPFTSFSVPDPELIKTVTINASSSRSGLDDINPTVLLKERSTEL
Inhibitor
Name:
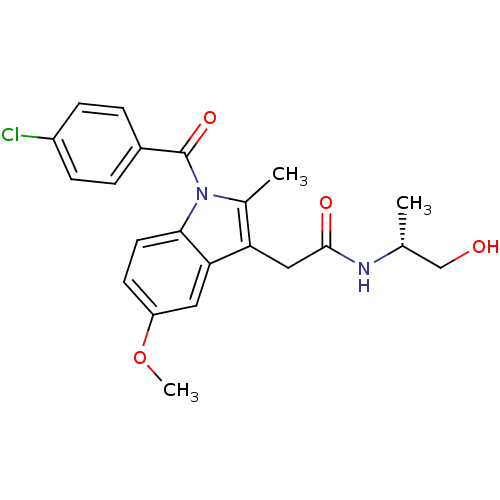
BDBM22569
Synonyms:
2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1H-indol-3-yl}-N-[(2R)-1-hydroxypropan-2-yl]acetamide | CHEMBL24874 | alpha-substituted indomethacin ethanolamide, 6
Type:
Small organic molecule
Emp. Form.:
C22H23ClN2O4
Mol. Mass.:
414.882
SMILES:
COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(=O)N[C@H](C)CO)c2c1 |r|