Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Botulinum neurotoxin type A2 [1-425]
Ligand
BDBM23269
Substrate
SNAPtide
Meas. Tech.
Evaluation of Inhibitors with Recombinant LC/A
pH
7.4±n/a
Temperature
295.65±n/a K
IC50
79000±8000 nM
Citation
 Boldt, GE; Kennedy, JP; Janda, KD Identification of a potent botulinum neurotoxin a protease inhibitor using in situ lead identification chemistry. Org Lett 8:1729-32 (2006) [PubMed] Article
Boldt, GE; Kennedy, JP; Janda, KD Identification of a potent botulinum neurotoxin a protease inhibitor using in situ lead identification chemistry. Org Lett 8:1729-32 (2006) [PubMed] Article Target
Name:
Botulinum neurotoxin type A2 [1-425]
Synonyms:
BXA2_CLOBJ | BoNT/A LC | Botulinum Neurotoxin Type A | Botulinum neurotoxin A light chain | atx | bna | bonT | bont/a2 | botA
Type:
Metalloprotease
Mol. Mass.:
48687.43
Organism:
Clostridium botulinum
Description:
Recombinant C. botulinum LC/A (1-425) was expressed in E. coli.
Residue:
425
Sequence:
MPFVNKQFNYKDPVNGVDIAYIKIPNAGQMQPVKAFKIHNKIWVIPERDTFTNPEEGDLNPPPEAKQVPVSYYDSTYLSTDNEKDNYLKGVTKLFERIYSTDLGRMLLTSIVRGIPFWGGSTIDTELKVIDTNCINVIQPDGSYRSEELNLVIIGPSADIIQFECKSFGHDVLNLTRNGYGSTQYIRFSPDFTFGFEESLEVDTNPLLGAGKFATDPAVTLAHELIHAEHRLYGIAINPNRVFKVNTNAYYEMSGLEVSFEELRTFGGHDAKFIDSLQENEFRLYYYNKFKDVASTLNKAKSIIGTTASLQYMKNVFKEKYLLSEDTSGKFSVDKLKFDKLYKMLTEIYTEDNFVNFFKVINRKTYLNFDKAVFRINIVPDENYTIKDGFNLKGANLSTNFNGQNTEINSRNFTRLKNFTGLFEF
Inhibitor
Name:
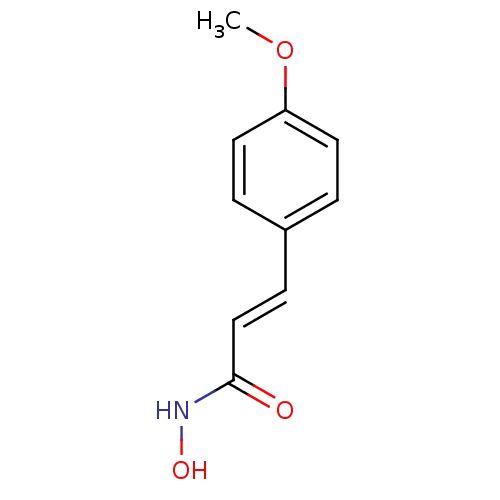
BDBM23269
Synonyms:
(2E)-N-hydroxy-3-(4-methoxyphenyl)prop-2-enamide | Cinnamic hydroxamate deriv., 13 | US10188756, Compound CN87 | US11505523, Compound PCI34051
Type:
Small organic molecule
Emp. Form.:
C10H11NO3
Mol. Mass.:
193.1992
SMILES:
COc1ccc(\C=C\C(=O)NO)cc1
Substrate
Name:
SNAPtide
Synonyms:
n/a
Type:
Fluorogenic Substrate
Mol. Mass.:
358.43
Organism:
n/a
Description:
Peptide derived from synaptosomes associated protein, SNAP-25. (U.S. Patent #6504006. List Biological Laboratories, Inc., Campbell, CA.).
Residue:
3
Sequence:
NA