Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 2A
Ligand
BDBM50207156
Substrate
n/a
Meas. Tech.
5-HT2A Receptor Binding Test
pH
7.7±n/a
Ki
0.290±n/a nM
Comments
extracted
Citation
More Info.:
Target
Name:
5-hydroxytryptamine receptor 2A
Synonyms:
5-HT-2 | 5-HT-2A | 5-HT2A | 5-hydroxytryptamine receptor 2A (5-HT-2A) | 5-hydroxytryptamine receptor 2A (5HT-2A) | 5-hydroxytryptamine receptor 2A (5HT2A) | 5HT2A_HUMAN | HTR2 | HTR2A | Serotonin receptor 2A
Type:
undefined
Mol. Mass.:
52607.65
Organism:
Homo sapiens (Human)
Description:
P28223
Residue:
471
Sequence:
MDILCEENTSLSSTTNSLMQLNDDTRLYSNDFNSGEANTSDAFNWTVDSENRTNLSCEGCLSPSCLSLLHLQEKNWSALLTAVVIILTIAGNILVIMAVSLEKKLQNATNYFLMSLAIADMLLGFLVMPVSMLTILYGYRWPLPSKLCAVWIYLDVLFSTASIMHLCAISLDRYVAIQNPIHHSRFNSRTKAFLKIIAVWTISVGISMPIPVFGLQDDSKVFKEGSCLLADDNFVLIGSFVSFFIPLTIMVITYFLTIKSLQKEATLCVSDLGTRAKLASFSFLPQSSLSSEKLFQRSIHREPGSYTGRRTMQSISNEQKACKVLGIVFFLFVVMWCPFFITNIMAVICKESCNEDVIGALLNVFVWIGYLSSAVNPLVYTLFNKTYRSAFSRYIQCQYKENKKPLQLILVNTIPALAYKSSQLQMGQKKNSKQDAKTTDNDCSMVALGKQHSEEASKDNSDGVNEKVSCV
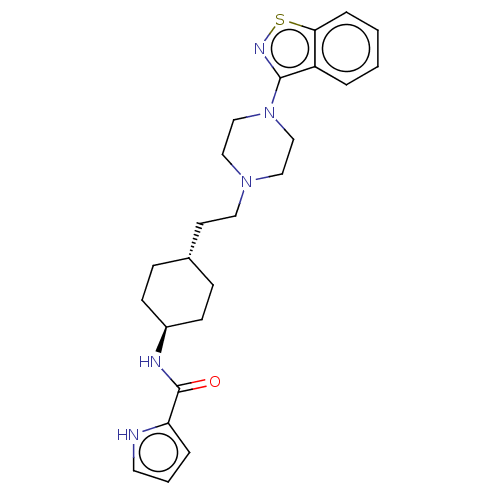
Inhibitor
Name:
BDBM50207156
Synonyms:
CHEMBL3938210 | US9550741, I-3
Type:
Small organic molecule
Emp. Form.:
C24H31N5OS
Mol. Mass.:
437.601
SMILES:
O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1ccc[nH]1 |r,wU:3.2,wD:6.6,(22.96,-24.14,;22.24,-22.76,;23.01,-21.42,;24.55,-21.42,;25.32,-20.09,;26.86,-20.09,;27.63,-21.42,;29.17,-21.42,;29.94,-20.09,;31.48,-20.09,;32.25,-21.42,;33.79,-21.42,;34.56,-20.09,;33.79,-18.75,;32.25,-18.75,;36.1,-20.09,;37.02,-21.32,;38.46,-20.86,;38.46,-19.32,;39.65,-18.3,;39.29,-16.8,;37.85,-16.29,;36.72,-17.32,;37.02,-18.86,;26.86,-22.76,;25.32,-22.76,;20.7,-22.66,;19.88,-21.38,;18.39,-21.79,;18.29,-23.33,;19.73,-23.89,)|