Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
3-hydroxy-3-methylglutaryl-coenzyme A reductase
Ligand
BDBM50011032
Substrate
n/a
Meas. Tech.
ChEMBL_80648 (CHEMBL691934)
IC50
25.0±n/a nM
Citation
 Roth, BD; Blankley, CJ; Chucholowski, AW; Ferguson, E; Hoefle, ML; Ortwine, DF; Newton, RS; Sekerke, CS; Sliskovic, DR; Stratton, CD Inhibitors of cholesterol biosynthesis. 3. Tetrahydro-4-hydroxy-6-[2-(1H-pyrrol-1-yl)ethyl]-2H-pyran-2-one inhibitors of HMG-CoA reductase. 2. Effects of introducing substituents at positions three and four of the pyrrole nucleus. J Med Chem 34:357-66 (1991) [PubMed] Article
Roth, BD; Blankley, CJ; Chucholowski, AW; Ferguson, E; Hoefle, ML; Ortwine, DF; Newton, RS; Sekerke, CS; Sliskovic, DR; Stratton, CD Inhibitors of cholesterol biosynthesis. 3. Tetrahydro-4-hydroxy-6-[2-(1H-pyrrol-1-yl)ethyl]-2H-pyran-2-one inhibitors of HMG-CoA reductase. 2. Effects of introducing substituents at positions three and four of the pyrrole nucleus. J Med Chem 34:357-66 (1991) [PubMed] Article More Info.:
Target
Name:
3-hydroxy-3-methylglutaryl-coenzyme A reductase
Synonyms:
HMDH_RAT | HMG-CoA reductase | Hmgcr
Type:
Enzyme
Mol. Mass.:
96689.85
Organism:
Rattus norvegicus (rat)
Description:
Isolated rat liver microsomes were used as enzyme source.
Residue:
887
Sequence:
MLSRLFRMHGLFVASHPWEVIVGTVTLTICMMSMNMFTGNNKICGWNYECPKFEEDVLSSDIIILTITRCIAILYIYFQFQNLRQLGSKYILGIAGLFTIFSSFVFSTVVIHFLDKELTGLNEALPFFLLLIDLSRASALAKFALSSNSQDEVRENIARGMAILGPTFTLDALVECLVIGVGTMSGVRQLEIMCCFGCMSVLANYFVFMTFFPACVSLVLELSRESREGRPIWQLSHFARVLEEEENKPNPVTQRVKMIMSLGLVLVHAHSRWIADPSPQNSTAEQSKVSLGLAEDVSKRIEPSVSLWQFYLSKMISMDIEQVITLSLALLLAVKYIFFEQAETESTLSLKNPITSPVVTPKKAQDNCCRREPLLVRRNQKLSSVEEDPGVNQDRKVEVIKPLVAEAETSGRATFVLGASAASPPLALGAQEPGIELPSEPRPNEECLQILESAEKGAKFLSDAEIIQLVNAKHIPAYKLETLMETHERGVSIRRQLLSAKLAEPSSLQYLPYRDYNYSLVMGACCENVIGYMPIPVGVAGPLCLDGKEYQVPMATTEGCLVASTNRGCRAISLGGGASSRVLADGMSRGPVVRLPRACDSAEVKSWLETPEGFAVVKEAFDSTSRFARLQKLHVTLAGRNLYIRLQSKTGDAMGMNMISKGTEKALLKLQEFFPELQILAVSGNYCTDKKPAAINWIEGRGKTVVCEAVIPAKVVREVLKTTTEAMVDVNINKNLVGSAMAGSIGGYNAHAANIVTAIYIACGQDAAQNVGSSNCITLMEASGPTNEDLYISCTMPSIEIGTVGGGTNLLPQQACLQMLGVQGACKDNPGENARQLARIVCGTVMAGELSLMAALAAGHLVRSHMVHNRSKINLQDLQGTCTKKAA
Inhibitor
Name:
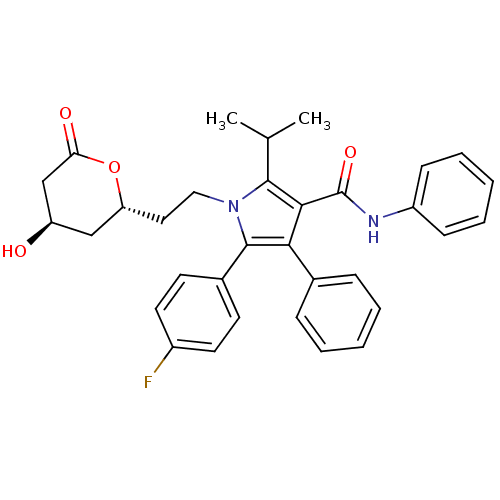
BDBM50011032
Synonyms:
5-(4-Fluoro-phenyl)-1-[2-(4-hydroxy-6-oxo-tetrahydro-pyran-2-yl)-ethyl]-2-isopropyl-4-phenyl-1H-pyrrole-3-carboxylic acid phenylamide | Atorvastatin lactone | CHEMBL333687 | US9006282, Example 3, Compound 2
Type:
Small organic molecule
Emp. Form.:
C33H33FN2O4
Mol. Mass.:
540.6245
SMILES:
CC(C)c1c(C(=O)Nc2ccccc2)c(c(-c2ccc(F)cc2)n1CC[C@@H]1C[C@@H](O)CC(=O)O1)-c1ccccc1