Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cholecystokinin receptor type A
Ligand
BDBM50016425
Substrate
n/a
Meas. Tech.
ChEBML_49556
Ki
4.3±n/a nM
Citation
 Charpentier, B; Durieux, C; Menant, I; Roques, BP Investigation of peripheral cholecystokinin receptor heterogeneity by cyclic and related linear analogues of CCK26-33: synthesis and biological properties. J Med Chem 30:962-8 (1987) [PubMed] Article
Charpentier, B; Durieux, C; Menant, I; Roques, BP Investigation of peripheral cholecystokinin receptor heterogeneity by cyclic and related linear analogues of CCK26-33: synthesis and biological properties. J Med Chem 30:962-8 (1987) [PubMed] Article More Info.:
Target
Name:
Cholecystokinin receptor type A
Synonyms:
CCKAR_RAT | Cckar | Cholecystokinin peripheral | Cholecystokinin receptor | Cholecystokinin receptor type A
Type:
Enzyme Catalytic Domain
Mol. Mass.:
49676.37
Organism:
RAT
Description:
Cholecystokinin central 0 RAT::P30551
Residue:
444
Sequence:
MSHSPARQHLVESSRMDVVDSLLMNGSNITPPCELGLENETLFCLDQPQPSKEWQSALQILLYSIIFLLSVLGNTLVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLKDFIFGSAVCKTTTYFMGTSVSVSTFNLVAISLERYGAICRPLQSRVWQTKSHALKVIAATWCLSFTIMTPYPIYSNLVPFTKNNNQTANMCRFLLPSDAMQQSWQTFLLLILFLLPGIVMVVAYGLISLELYQGIKFDASQKKSAKEKKPSTGSSTRYEDSDGCYLQKSRPPRKLELQQLSSGSGGSRLNRIRSSSSAANLIAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTVSAEKHLSGTPISFILLLSYTSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGVRGEVGEEEDGRTIRALLSRYSYSHMSTSAPPP
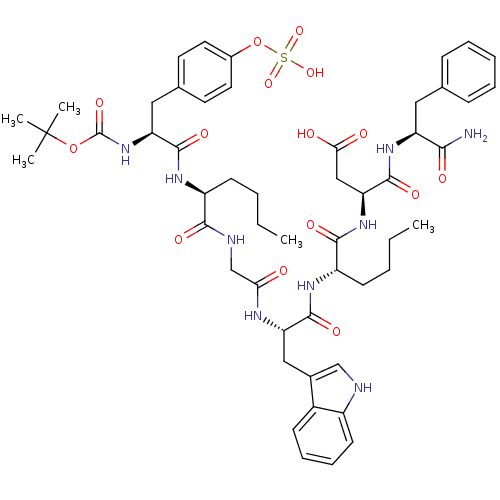
Inhibitor
Name:
BDBM50016425
Synonyms:
(S)-3-{(S)-2-[(S)-2-(2-{(S)-2-[(S)-2-tert-Butoxycarbonylamino-3-(4-sulfooxy-phenyl)-propionylamino]-hexanoylamino}-acetylamino)-3-(1H-indol-3-yl)-propionylamino]-hexanoylamino}-N-((S)-1-carbamoyl-2-phenyl-ethyl)-succinamic acid | 3-{2-[2-(2-{2-[2-(tert-Butoxycarbonyl-methyl-amino)-3-(4-sulfonyl-oxy-phenyl)-propionylamino]-hexanoylamino}-acetylamino)-3-(1H-indol-3-yl)-propionylamino]-hexanoylamino}-N-(1-carbamoyl-2-phenyl-ethyl)-succinamic acid | 3-{2-[2-(2-{2-[2-tert-Butoxycarbonylamino-3-(4-sulfooxy-phenyl)-propionylamino]-hexanoylamino}-acetylamino)-3-(1H-indol-3-yl)-propionylamino]-hexanoylamino}-N-(1-carbamoyl-2-phenyl-ethyl)-succinamic acid | Boc-Tyr(SO3H)-Nle-Gly-Trp-Nle-Asp-Phe-NH2 | CHEMBL384035
Type:
Small organic molecule
Emp. Form.:
C52H69N9O15S
Mol. Mass.:
1092.221
SMILES:
CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O