Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Alpha-mannosidase 2
Ligand
BDBM18351
Substrate
n/a
Meas. Tech.
ChEMBL_32497 (CHEMBL641374)
Ki
<50000±n/a nM
Citation
 Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem 38:2349-56 (1995) [PubMed] Article
Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem 38:2349-56 (1995) [PubMed] Article More Info.:
Target
Name:
Alpha-mannosidase 2
Synonyms:
MA2A1_RAT | Man2a1 | Mana2 | Mannosidase 2 alpha 1
Type:
PROTEIN
Mol. Mass.:
131265.36
Organism:
Rattus norvegicus
Description:
ChEMBL_32391
Residue:
1148
Sequence:
MKLSRQFTVFGSAIFCVVIFSLYLMLDRGHLDYPRGPRQEGSFPQGQLSILQEKIDHLERLLAENNEIISNIRDSVINLSESVEDGPRGPAGNASQGSAHLHSAQLALQADPKDCLFASQSGNQHRDVQMLDVYDLIPFDNPDGGVWKQGFDIKYEADEWDREPLQVFVVPHSHNDPGWLKTFNDYFRDKTQYIFNNMVLKLKEDSSRKFIWSEISYLAKWWDIIDNPKKEAVKSLLQNGQLEIVTGGWVMADEATTHYFALIDQLIEGHQWLEKNLGVKPRSGWAIDPFGHSPTMTYLLKRAGFSHMLIQRVHYSVKKHFSLQKTLEFFWRQNWDLGSTTDILCHMMPFYSYDIPHTCGPDPKICCQFDFKRLPGGRYGCPWGVPPEAISPGNVQSRAQMLLDQYRKKSKLFRTKVLLAPLGDDFRFSEYTEWDLQYRNYEQLFSYMNSQPHLKVKIQFGTLSDYFDALEKSVAAEKKGGQSVFPALSGDFFTYADRDDHYWSGYFTSRPFYKRMDRIMESRLRTAEILYHLALKQAQKYKINKFLSSPHYTTLTEARRNLGLFQHHDAITGTAKDWVVVDYGTRLFQSLNSLEKIIGDSAFLLILKDKKLYQSDPSKAFLEMDTKQSSQDSLPKKNIIQLSAQEPRYLVVYNPFEQERHSVVSVRVNSATVKVLSDLGKAVEVQVSAVWKDMRTTSQAAYEVAFLAHLPPLGLKVYKILESQSSSSHLADYFLYNNDGQAESGIFHMKNMVDSGDAITIENSFLTLGFDRSGLMEKVRRKEDNKQQELKVQFLWYGTTNKRDKSGAYLFLPDGQGQPYVSLRTPFVRVTRGRIYSDVTCFLEHVTHKVRLYHIQGIEGQSMEVSNIVDIRSVHNREIVMRISSKINNQNRYYTDLNGYQIQPRRTMAKLPLQANVYPMSTMAYIQDAAHRLTLLSAQSLGASSMASGQIEVFMDRRLMQDDNRGLGQGVHDNKITANLFRILLEKRNGMNMEEDKKSPVSYPSLLSHMTSAFLNHPFLPMVLSGQLPSPAIELLSEFRLLQSSLPCDIHLVNLRTIQSKVGKGYSDEAALILHRKVFDCQLSSRAMGLPCSTTQGKMSIPKLFNNFAVESFIPSSLSLMHSPPDAQNTSEVSLSPMEISTSRIRLR
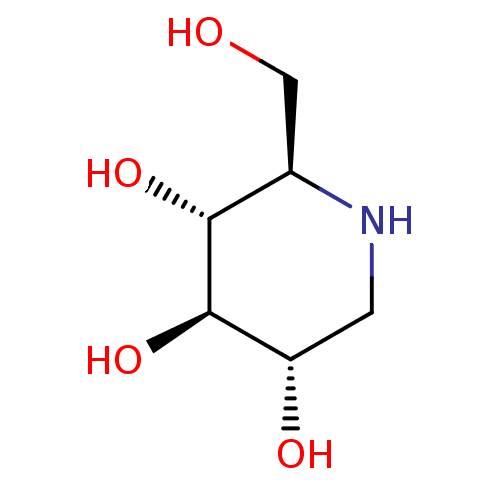
Inhibitor
Name:
BDBM18351
Synonyms:
(2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-triol, 10 | (2R,3R,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-triol | 1-Deoxynojirimycin | 1-deoxynojirimycin (DNJ) | CHEMBL307429 | US20230339856, Compound DNJ | US9181184, 1 | dNM
Type:
natural product
Emp. Form.:
C6H13NO4
Mol. Mass.:
163.1717
SMILES:
OC[C@H]1NC[C@H](O)[C@@H](O)[C@@H]1O