Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Reverse transcriptase
Ligand
BDBM50492151
Substrate
n/a
Meas. Tech.
ChEMBL_968173 (CHEMBL2399276)
IC50
5.5±n/a nM
Citation
 Sturino, CF; Bousquet, Y; James, CA; DeRoy, P; Duplessis, M; Edwards, PJ; Halmos, T; Minville, J; Morency, L; Morin, S; Thavonekham, B; Tremblay, M; Duan, J; Ribadeneira, M; Garneau, M; Pelletier, A; Tremblay, S; Lamorte, L; Bethell, R; Cordingley, MG; Rajotte, D; Simoneau, B Identification of potent and orally bioavailable nucleotide competing reverse transcriptase inhibitors: in vitro and in vivo optimization of a series of benzofurano[3,2-d]pyrimidin-2-one derived inhibitors. Bioorg Med Chem Lett 23:3967-75 (2013) [PubMed] Article
Sturino, CF; Bousquet, Y; James, CA; DeRoy, P; Duplessis, M; Edwards, PJ; Halmos, T; Minville, J; Morency, L; Morin, S; Thavonekham, B; Tremblay, M; Duan, J; Ribadeneira, M; Garneau, M; Pelletier, A; Tremblay, S; Lamorte, L; Bethell, R; Cordingley, MG; Rajotte, D; Simoneau, B Identification of potent and orally bioavailable nucleotide competing reverse transcriptase inhibitors: in vitro and in vivo optimization of a series of benzofurano[3,2-d]pyrimidin-2-one derived inhibitors. Bioorg Med Chem Lett 23:3967-75 (2013) [PubMed] Article More Info.:
Target
Name:
Reverse transcriptase
Synonyms:
n/a
Type:
Protein
Mol. Mass.:
29598.37
Organism:
Human immunodeficiency virus 1
Description:
Q9WKE8
Residue:
254
Sequence:
PISPITVPVKLKPGMDGPKVKQWPLTEEKIKALTEICTEMEKEGKIEKIGPENPYNTPVFAIKKKDSTKWRKVVDFRELNKRTQDFWEVQLGIPHPAGLKKKKSVTVLDVGDAYFSVPLDKDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVIYQYMDDLYVGSDLEIEQHRAKIEELRQHLLRWGFTTPDKKHQKEPPFLWMGYELHPDKWTVQPIVLPEKDSWTVN
Inhibitor
Name:
BDBM50492151
Synonyms:
CHEMBL2397588
Type:
Small organic molecule
Emp. Form.:
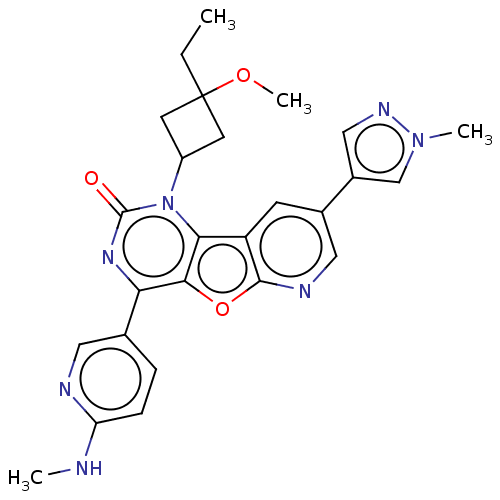
C26H27N7O3
Mol. Mass.:
485.5377
SMILES:
CCC1(CC(C1)n1c2c3cc(cnc3oc2c(nc1=O)-c1ccc(NC)nc1)-c1cnn(C)c1)OC |(52.36,-18.14,;51.27,-19.22,;51.67,-20.72,;53.19,-20.95,;52.96,-22.47,;51.44,-22.24,;53.87,-23.71,;53.25,-25.13,;51.78,-25.61,;50.44,-24.85,;49.11,-25.62,;49.11,-27.16,;50.44,-27.93,;51.78,-27.16,;53.25,-27.64,;54.17,-26.38,;55.71,-26.22,;56.33,-24.8,;55.42,-23.54,;56.04,-22.13,;56.61,-27.45,;55.99,-28.86,;56.9,-30.1,;58.43,-29.94,;59.34,-31.18,;60.87,-31.01,;59.05,-28.52,;58.14,-27.28,;47.79,-24.84,;46.38,-25.47,;45.35,-24.32,;46.12,-22.99,;45.49,-21.58,;47.62,-23.31,;50.17,-20.31,;49.78,-18.83,)|