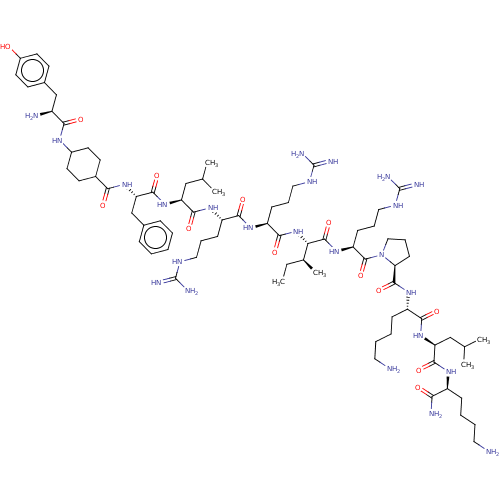
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Delta-type opioid receptor
Ligand
BDBM50040188
Substrate
n/a
Meas. Tech.
ChEMBL_147052 (CHEMBL753279)
Ki
1370±n/a nM
Citation
 Snyder, KR; Murray, TF; DeLander, GE; Aldrich, JV Synthesis and opioid activity of dynorphin A-(1-13)NH2 analogues containing cis- and trans-4-aminocyclohexanecarboxylic acid. J Med Chem 36:1100-3 (1993) [PubMed] Article
Snyder, KR; Murray, TF; DeLander, GE; Aldrich, JV Synthesis and opioid activity of dynorphin A-(1-13)NH2 analogues containing cis- and trans-4-aminocyclohexanecarboxylic acid. J Med Chem 36:1100-3 (1993) [PubMed] Article More Info.:
Target
Name:
Delta-type opioid receptor
Synonyms:
Cytochrome P450 3A4 | DOR-1 | Delta opioid receptor | Delta-type opioid receptor | Delta-type opioid receptor (DOR) | OPIATE Delta | OPRD_RAT | Opiate Delta 1 | Opioid receptor | Opioid receptor A | Opioid receptors; mu & delta | Oprd1 | Ror-a | Voltage-gated potassium channel
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
40465.04
Organism:
Rattus norvegicus (rat)
Description:
Competition binding assays were using CHO-K1 cell membranes expressing the opioid receptor.
Residue:
372
Sequence:
MEPVPSARAELQFSLLANVSDTFPSAFPSASANASGSPGARSASSLALAIAITALYSAVCAVGLLGNVLVMFGIVRYTKLKTATNIYIFNLALADALATSTLPFQSAKYLMETWPFGELLCKAVLSIDYYNMFTSIFTLTMMSVDRYIAVCHPVKALDFRTPAKAKLINICIWVLASGVGVPIMVMAVTQPRDGAVVCTLQFPSPSWYWDTVTKICVFLFAFVVPILIITVCYGLMLLRLRSVRLLSGSKEKDRSLRRITRMVLVVVGAFVVCWAPIHIFVIVWTLVDINRRDPLVVAALHLCIALGYANSSLNPVLYAFLDENFKRCFRQLCRAPCGGQEPGSLRRPRQATARERVTACTPSDGPGGGAAA
Inhibitor
Name:
BDBM50040188
Synonyms:
(cis)Tyr-1-Amino-CycloHexylcarbonyl-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-NH2 | (trans)Tyr-1-Amino-CycloHexylcarbonyl-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-NH2 | CHEMBL2369477
Type:
Small organic molecule
Emp. Form.:
C78H132N24O13
Mol. Mass.:
1614.0357
SMILES:
CC[C@H](C)[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)C1CCC(CC1)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(N)=O |wU:4.4,98.101,106.109,38.38,19.19,wD:85.89,71.73,89.92,30.30,58.61,8.8,2.2,(17.35,.41,;17.35,-.83,;18.68,-1.6,;19.75,-.98,;18.68,-3.14,;17.34,-3.9,;16.01,-3.13,;16.01,-1.9,;14.67,-3.89,;14.66,-5.43,;13.33,-6.2,;13.32,-7.74,;11.98,-8.5,;11.97,-10.04,;10.9,-10.66,;13.03,-10.66,;13.34,-3.12,;12,-3.88,;12,-5.12,;10.67,-3.11,;10.67,-1.57,;9.34,-.8,;9.34,.74,;8.01,1.52,;8.01,3.06,;6.95,3.68,;9.08,3.67,;9.33,-3.88,;8,-3.1,;8,-1.87,;6.66,-3.87,;6.66,-5.41,;5.32,-6.17,;4.25,-5.55,;5.31,-7.4,;5.33,-3.09,;4,-3.86,;3.99,-5.09,;2.66,-3.08,;2.67,-1.54,;4,-.77,;4,.77,;5.34,1.54,;6.67,.76,;6.66,-.78,;5.33,-1.54,;1.33,-3.85,;-.01,-3.07,;-0,-1.84,;-1.34,-3.84,;-2.68,-3.07,;-4.01,-3.83,;-4.01,-5.37,;-2.68,-6.15,;-1.35,-5.38,;-5.35,-6.14,;-5.35,-7.69,;-4.28,-8.3,;-6.68,-8.46,;-7.75,-7.84,;-6.68,-10,;-8.01,-10.77,;-8.02,-12.31,;-9.35,-13.08,;-10.69,-12.3,;-11.75,-12.92,;-10.68,-10.76,;-9.35,-10,;20.01,-3.91,;20.01,-5.15,;21.35,-3.15,;22.68,-3.92,;22.67,-5.46,;21.33,-6.22,;21.32,-7.77,;19.99,-8.53,;19.98,-10.07,;18.91,-10.68,;21.04,-10.69,;24.02,-3.16,;24.02,-1.92,;25.35,-3.93,;25.48,-5.45,;26.99,-5.78,;27.76,-4.45,;26.74,-3.3,;27.05,-1.8,;28.22,-1.41,;25.9,-.77,;26.22,.74,;27.68,1.22,;28.83,.19,;30.3,.67,;31.44,-.36,;32.61,.03,;25.07,1.77,;23.9,1.38,;25.38,3.28,;24.23,4.3,;22.77,3.82,;22.46,2.31,;21.29,1.92,;23.38,1.49,;24.55,5.8,;25.71,6.19,;23.4,6.83,;23.71,8.34,;22.56,9.37,;22.87,10.88,;21.73,11.91,;22.04,13.41,;21.12,14.23,;25.17,8.83,;26.09,8.01,;25.42,10.04,)|