Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50121975
Substrate
n/a
Meas. Tech.
ChEMBL_1877247 (CHEMBL4378641)
IC50
28000±n/a nM
Citation
 Chatzopoulou, M; Claridge, TDW; Davies, KE; Davies, SG; Elsey, DJ; Emer, E; Fletcher, AM; Harriman, S; Robinson, N; Rowley, JA; Russell, AJ; Tinsley, JM; Weaver, R; Wilkinson, IVL; Willis, NJ; Wilson, FX; Wynne, GM Isolation, Structural Identification, Synthesis, and Pharmacological Profiling of 1,2- J Med Chem 63:2547-2556 (2020) [PubMed] Article
Chatzopoulou, M; Claridge, TDW; Davies, KE; Davies, SG; Elsey, DJ; Emer, E; Fletcher, AM; Harriman, S; Robinson, N; Rowley, JA; Russell, AJ; Tinsley, JM; Weaver, R; Wilkinson, IVL; Willis, NJ; Wilson, FX; Wynne, GM Isolation, Structural Identification, Synthesis, and Pharmacological Profiling of 1,2- J Med Chem 63:2547-2556 (2020) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
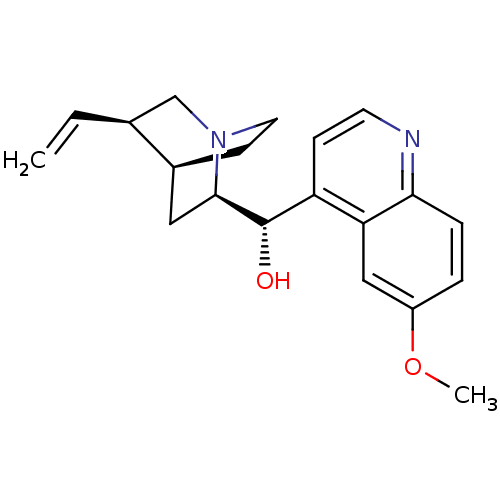
BDBM50121975
Synonyms:
(6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2.2.2]oct-2-yl)-methanol | CARDIOQUIN | CHEMBL1294 | CIN-QUIN | DURAQUIN | QUINACT | QUINAGLUTE | QUINALAN | QUINATIME | QUINIDEX | QUINIDINE | QUINORA | US9402878, Quinidine
Type:
Small organic molecule
Emp. Form.:
C20H24N2O2
Mol. Mass.:
324.4168
SMILES:
COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15|