Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Bifunctional dihydrofolate reductase-thymidylate synthase
Ligand
BDBM50110752
Substrate
n/a
Meas. Tech.
ChEMBL_55400 (CHEMBL668409)
Ki
5.2±n/a nM
Citation
 Tarnchompoo, B; Sirichaiwat, C; Phupong, W; Intaraudom, C; Sirawaraporn, W; Kamchonwongpaisan, S; Vanichtanankul, J; Thebtaranonth, Y; Yuthavong, Y Development of 2,4-diaminopyrimidines as antimalarials based on inhibition of the S108N and C59R+S108N mutants of dihydrofolate reductase from pyrimethamine-resistant Plasmodium falciparum. J Med Chem 45:1244-52 (2002) [PubMed] Article
Tarnchompoo, B; Sirichaiwat, C; Phupong, W; Intaraudom, C; Sirawaraporn, W; Kamchonwongpaisan, S; Vanichtanankul, J; Thebtaranonth, Y; Yuthavong, Y Development of 2,4-diaminopyrimidines as antimalarials based on inhibition of the S108N and C59R+S108N mutants of dihydrofolate reductase from pyrimethamine-resistant Plasmodium falciparum. J Med Chem 45:1244-52 (2002) [PubMed] Article More Info.:
Target
Name:
Bifunctional dihydrofolate reductase-thymidylate synthase
Synonyms:
DHFR-TS | DRTS_PLAFK | Dihydrofolate Reductase-Thymidylate Synthase (DHFR-TS) Mutant KICB1 | Dihydrofolate reductase | PfDHFR-TS double mutant (C59R+S108N)
Type:
Enzyme
Mol. Mass.:
71822.51
Organism:
Plasmodium falciparum (isolate K1 / Thailand)
Description:
The mutant clone was prepared by cassette mutagenesis using wildtype pfDHFR as a template, and expressed in E. coli.
Residue:
608
Sequence:
MMEQVCDVFDIYAICACCKVESKNEGKKNEVFNNYTFRGLGNKGVLPWKCNSLDMKYFRAVTTYVNESKYEKLKYKRCKYLNKETVDNVNDMPNSKKLQNVVVMGRTNWESIPKKFKPLSNRINVILSRTLKKEDFDEDVYIINKVEDLIVLLGKLNYYKCFIIGGSVVYQEFLEKKLIKKIYFTRINSTYECDVFFPEINENEYQIISVSDVYTSNNTTLDFIIYKKTNNKMLNEQNCIKGEEKNNDMPLKNDDKDTCHMKKLTEFYKNVDKYKINYENDDDDEEEDDFVYFNFNKEKEEKNKNSIHPNDFQIYNSLKYKYHPEYQYLNIIYDIMMNGNKQSDRTGVGVLSKFGYIMKFDLSQYFPLLTTKKLFLRGIIEELLWFIRGETNGNTLLNKNVRIWEANGTREFLDNRKLFHREVNDLGPIYGFQWRHFGAEYTNMYDNYENKGVDQLKNIINLIKNDPTSRRILLCAWNVKDLDQMALPPCHILCQFYVFDGKLSCIMYQRSCDLGLGVPFNIASYSIFTHMIAQVCNLQPAQFIHVLGNAHVYNNHIDSLKIQLNRIPYPFPTLKLNPDIKNIEDFTISDFTIQNYVHHEKISMDMAA
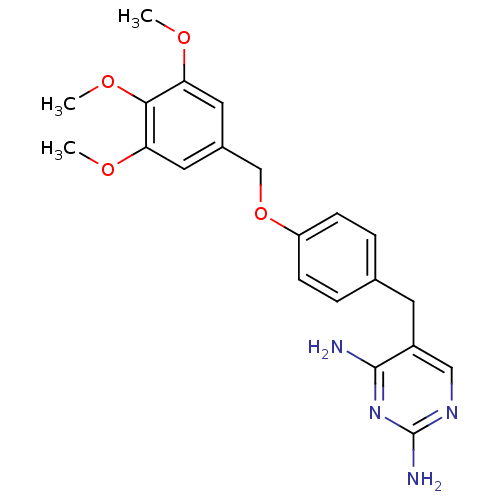
Inhibitor
Name:
BDBM50110752
Synonyms:
5-(4-(3,4,5-trimethoxybenzyloxy)benzyl)pyrimidine-2,4-diamine | 5-[4-(3,4,5-Trimethoxy-benzyloxy)-benzyl]-pyrimidine-2,4-diamine | CHEMBL416376
Type:
Small organic molecule
Emp. Form.:
C21H24N4O4
Mol. Mass.:
396.4397
SMILES:
COc1cc(COc2ccc(Cc3cnc(N)nc3N)cc2)cc(OC)c1OC