Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Inosine-5'-monophosphate dehydrogenase 2
Ligand
BDBM50133712
Substrate
n/a
Meas. Tech.
ChEBML_89932
IC50
260±n/a nM
Citation
 Dhar, TG; Shen, Z; Gu, HH; Chen, P; Norris, D; Watterson, SH; Ballentine, SK; Fleener, CA; Rouleau, KA; Barrish, JC; Townsend, R; Hollenbaugh, DL; Iwanowicz, EJ 3-cyanoindole-based inhibitors of inosine monophosphate dehydrogenase: synthesis and initial structure-activity relationships. Bioorg Med Chem Lett 13:3557-60 (2003) [PubMed] Article
Dhar, TG; Shen, Z; Gu, HH; Chen, P; Norris, D; Watterson, SH; Ballentine, SK; Fleener, CA; Rouleau, KA; Barrish, JC; Townsend, R; Hollenbaugh, DL; Iwanowicz, EJ 3-cyanoindole-based inhibitors of inosine monophosphate dehydrogenase: synthesis and initial structure-activity relationships. Bioorg Med Chem Lett 13:3557-60 (2003) [PubMed] Article More Info.:
Target
Name:
Inosine-5'-monophosphate dehydrogenase 2
Synonyms:
IMDH2_HUMAN | IMP dehydrogenase 2 | IMPD 2 | IMPD2 | IMPDH-II | IMPDH2 | Inosine 5'-monophosphate dehydrogenase II (IMPDH II) | Inosine Monophosphate Dehydrogenase Type 2 (IMPDH2) | Inosine-5 -monophosphate dehydrogenase 2 | Inosine-5'-monophosphate dehydrogenase (IMPDH)
Type:
Enzyme
Mol. Mass.:
55806.87
Organism:
Homo sapiens (Human)
Description:
Recombinant IMPDH2 expressed in E. coli.
Residue:
514
Sequence:
MADYLISGGTSYVPDDGLTAQQLFNCGDGLTYNDFLILPGYIDFTADQVDLTSALTKKITLKTPLVSSPMDTVTEAGMAIAMALTGGIGFIHHNCTPEFQANEVRKVKKYEQGFITDPVVLSPKDRVRDVFEAKARHGFCGIPITDTGRMGSRLVGIISSRDIDFLKEEEHDCFLEEIMTKREDLVVAPAGITLKEANEILQRSKKGKLPIVNEDDELVAIIARTDLKKNRDYPLASKDAKKQLLCGAAIGTHEDDKYRLDLLAQAGVDVVVLDSSQGNSIFQINMIKYIKDKYPNLQVIGGNVVTAAQAKNLIDAGVDALRVGMGSGSICITQEVLACGRPQATAVYKVSEYARRFGVPVIADGGIQNVGHIAKALALGASTVMMGSLLAATTEAPGEYFFSDGIRLKKYRGMGSLDAMDKHLSSQNRYFSEADKIKVAQGVSGAVQDKGSIHKFVPYLIAGIQHSCQDIGAKSLTQVRAMMYSGELKFEKRTSSAQVEGGVHSLHSYEKRLF
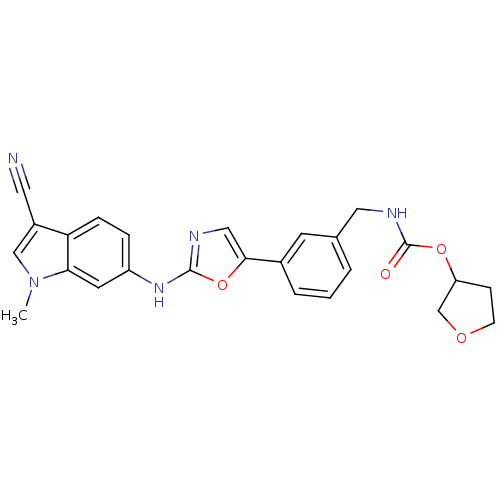
Inhibitor
Name:
BDBM50133712
Synonyms:
CHEMBL333662 | {3-[2-(3-Cyano-1-methyl-1H-indol-6-ylamino)-oxazol-5-yl]-benzyl}-carbamic acid tetrahydro-furan-3-yl ester
Type:
Small organic molecule
Emp. Form.:
C25H23N5O4
Mol. Mass.:
457.4812
SMILES:
Cn1cc(C#N)c2ccc(Nc3ncc(o3)-c3cccc(CNC(=O)OC4CCOC4)c3)cc12