Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Proteasome subunit beta type-1
Ligand
BDBM50069985
Substrate
n/a
Meas. Tech.
ChEMBL_353296 (CHEMBL861975)
Ki
470±n/a nM
Citation
 Rydzewski, RM; Burrill, L; Mendonca, R; Palmer, JT; Rice, M; Tahilramani, R; Bass, KE; Leung, L; Gjerstad, E; Janc, JW; Pan, L Optimization of subsite binding to the beta5 subunit of the human 20S proteasome using vinyl sulfones and 2-keto-1,3,4-oxadiazoles: syntheses and cellular properties of potent, selective proteasome inhibitors. J Med Chem 49:2953-68 (2006) [PubMed] Article
Rydzewski, RM; Burrill, L; Mendonca, R; Palmer, JT; Rice, M; Tahilramani, R; Bass, KE; Leung, L; Gjerstad, E; Janc, JW; Pan, L Optimization of subsite binding to the beta5 subunit of the human 20S proteasome using vinyl sulfones and 2-keto-1,3,4-oxadiazoles: syntheses and cellular properties of potent, selective proteasome inhibitors. J Med Chem 49:2953-68 (2006) [PubMed] Article More Info.:
Target
Name:
Proteasome subunit beta type-1
Synonyms:
PSB1_HUMAN | PSC5 | PSMB1 | Proteasome component C5 | Proteasome subunit beta type-1/beta type-5
Type:
PROTEIN
Mol. Mass.:
26493.62
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1366691
Residue:
241
Sequence:
MLSSTAMYSAPGRDLGMEPHRAAGPLQLRFSPYVFNGGTILAIAGEDFAIVASDTRLSEGFSIHTRDSPKCYKLTDKTVIGCSGFHGDCLTLTKIIEARLKMYKHSNNKAMTTGAIAAMLSTILYSRRFFPYYVYNIIGGLDEEGKGAVYSFDPVGSYQRDSFKAGGSASAMLQPLLDNQVGFKNMQNVEHVPLSLDRAMRLVKDVFISAAERDVYTGDALRICIVTKEGIREETVSLRKD
Inhibitor
Name:
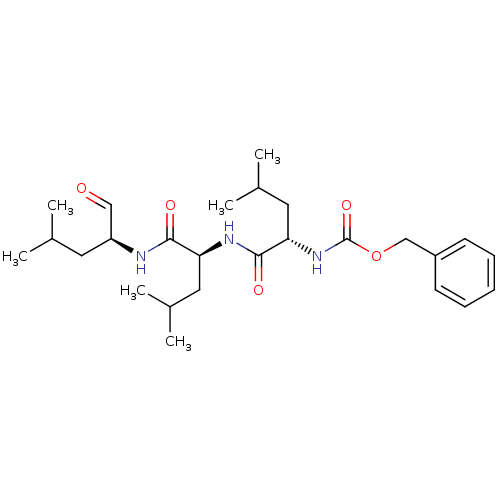
BDBM50069985
Synonyms:
(S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic acid [(S)-1-((S)-1-formyl-3-methyl-butylcarbamoyl)-3-methyl-butyl]-amide | CHEMBL64925 | Cbz-L-leu-L-leu-L-leu-CHO | MG-13 | MG-132 | Z-L-leu-L-leu-L-leu-H | Z-Leu-Leu-Leu-H | Z-Leu-Leu-Leu-al | benzyl (S)-4-methyl-1-((S)-4-methyl-1-((S)-4-methyl-1-oxopentan-2-ylamino)-1-oxopentan-2-ylamino)-1-oxopentan-2-ylcarbamate | benzyl(S)-4-methyl-1-((S)-4-methyl-1-((S)-4-methyl-1-oxopentan-2-ylamino)-1-oxopentan-2-ylamino)-1-oxopentan-2-ylcarbamate | benzyloxycarbonyl-Leu-Leu-leucinal | {(S)-1-[(S)-1-((S)-1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester | {1-[(S)-(S)-1-((S)-1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester | {1-[1-(1-Formyl-3-methyl-butylcarbamoyl)-3-methyl-butylcarbamoyl]-3-methyl-butyl}-carbamic acid benzyl ester
Type:
Small organic molecule
Emp. Form.:
C26H41N3O5
Mol. Mass.:
475.6208
SMILES:
CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r|