Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Lactoylglutathione lyase
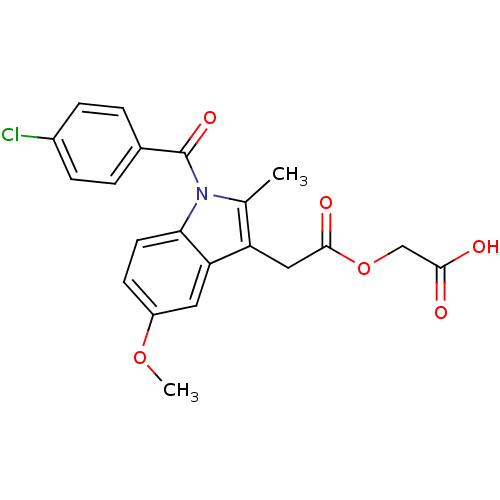
Ligand
BDBM50336272
Substrate
n/a
Meas. Tech.
ChEMBL_716548 (CHEMBL1670272)
Ki
49000±n/a nM
Citation
 Yuan, M; Luo, M; Song, Y; Xu, Q; Wang, X; Cao, Y; Bu, X; Ren, Y; Hu, X Identification of curcumin derivatives as human glyoxalase I inhibitors: A combination of biological evaluation, molecular docking, 3D-QSAR and molecular dynamics simulation studies. Bioorg Med Chem 19:1189-96 (2011) [PubMed] Article
Yuan, M; Luo, M; Song, Y; Xu, Q; Wang, X; Cao, Y; Bu, X; Ren, Y; Hu, X Identification of curcumin derivatives as human glyoxalase I inhibitors: A combination of biological evaluation, molecular docking, 3D-QSAR and molecular dynamics simulation studies. Bioorg Med Chem 19:1189-96 (2011) [PubMed] Article More Info.:
Target
Name:
Lactoylglutathione lyase
Synonyms:
Aldoketomutase | GLO1 | Glx I | Glyoxalase 1 (GLO1) | Glyoxalase I | Ketone-aldehyde mutase | LGUL_HUMAN | Methylglyoxalase | S-D-lactoylglutathione methylglyoxal lyase
Type:
Enzyme
Mol. Mass.:
20772.95
Organism:
Homo sapiens (Human)
Description:
Q04760
Residue:
184
Sequence:
MAEPQPPSGGLTDEAALSCCSDADPSTKDFLLQQTMLRVKDPKKSLDFYTRVLGMTLIQKCDFPIMKFSLYFLAYEDKNDIPKEKDEKIAWALSRKATLELTHNWGTEDDETQSYHNGNSDPRGFGHIGIAVPDVYSACKRFEELGVKFVKKPDDGKMKGLAFIQDPDGYWIEILNPNKMATLM