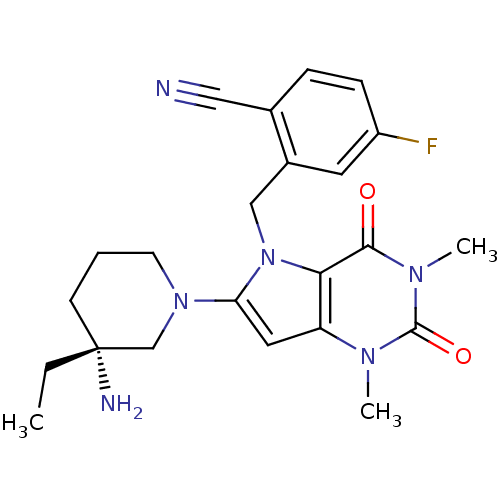
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50351401
Substrate
n/a
Meas. Tech.
ChEMBL_764492 (CHEMBL1821044)
Ki
0.022000±n/a nM
Citation
 Nishio, Y; Kimura, H; Sawada, N; Sugaru, E; Horiguchi, M; Ono, M; Furuta, Y; Sakai, M; Masui, Y; Otani, M; Hashizuka, T; Honda, Y; Deguchi, J; Nakagawa, T; Nakahira, H 2-({6-[(3R)-3-amino-3-methylpiperidine-1-yl]-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-5H-pyrrolo[3,2-d]pyrimidine-5-yl}methyl)-4-fluorobenzonitrile (DSR-12727): a potent, orally active dipeptidyl peptidase IV inhibitor without mechanism-based inactivation of CYP3A. Bioorg Med Chem 19:5490-9 (2011) [PubMed] Article
Nishio, Y; Kimura, H; Sawada, N; Sugaru, E; Horiguchi, M; Ono, M; Furuta, Y; Sakai, M; Masui, Y; Otani, M; Hashizuka, T; Honda, Y; Deguchi, J; Nakagawa, T; Nakahira, H 2-({6-[(3R)-3-amino-3-methylpiperidine-1-yl]-1,3-dimethyl-2,4-dioxo-1,2,3,4-tetrahydro-5H-pyrrolo[3,2-d]pyrimidine-5-yl}methyl)-4-fluorobenzonitrile (DSR-12727): a potent, orally active dipeptidyl peptidase IV inhibitor without mechanism-based inactivation of CYP3A. Bioorg Med Chem 19:5490-9 (2011) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA