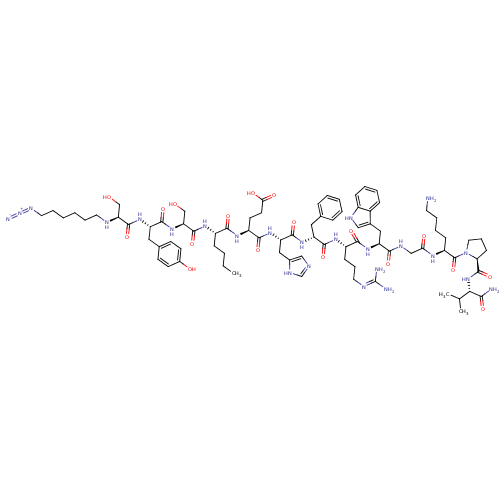
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Melanocyte-stimulating hormone receptor
Ligand
BDBM50354718
Substrate
n/a
Meas. Tech.
ChEMBL_771453 (CHEMBL1837213)
IC50
0.250000±n/a nM
Citation
 Baumhover, NJ; Martin, ME; Parameswarappa, SG; Kloepping, KC; O'Dorisio, MS; Pigge, FC; Schultz, MK Improved synthesis and biological evaluation of chelator-modifieda-MSH analogs prepared by copper-free click chemistry. Bioorg Med Chem Lett 21:5757-61 (2011) [PubMed] Article
Baumhover, NJ; Martin, ME; Parameswarappa, SG; Kloepping, KC; O'Dorisio, MS; Pigge, FC; Schultz, MK Improved synthesis and biological evaluation of chelator-modifieda-MSH analogs prepared by copper-free click chemistry. Bioorg Med Chem Lett 21:5757-61 (2011) [PubMed] Article More Info.:
Target
Name:
Melanocyte-stimulating hormone receptor
Synonyms:
MC1-R | MSHR_MOUSE | Mc1r | Melanocortin receptor 1 | Melanocyte-stimulating hormone receptor | Msh-r
Type:
PROTEIN
Mol. Mass.:
35238.60
Organism:
Mus musculus
Description:
ChEMBL_1498846
Residue:
315
Sequence:
MSTQEPQKSLLGSLNSNATSHLGLATNQSEPWCLYVSIPDGLFLSLGLVSLVENVLVVIAITKNRNLHSPMYYFICCLALSDLMVSVSIVLETTIILLLEAGILVARVALVQQLDNLIDVLICGSMVSSLCFLGIIAIDRYISIFYALRYHSIVTLPRARRAVVGIWMVSIVSSTLFITYYKHTAVLLCLVTFFLAMLALMAILYAHMFTRACQHAQGIAQLHKRRRSIRQGFCLKGAATLTILLGIFFLCWGPFFLHLLLIVLCPQHPTCSCIFKNFNLFLLLIVLSSTVDPLIYAFRSQELRMTLKEVLLCSW
Inhibitor
Name:
BDBM50354718
Synonyms:
CHEMBL1834392
Type:
Small organic molecule
Emp. Form.:
C82H120N24O18
Mol. Mass.:
1729.9802
SMILES:
CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NCCCCCCN=[N+]=[N-])C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:26.29,8.10,4.4,83.85,101.105,117.122,wD:14.23,42.42,51.51,61.62,72.74,113.119,(18.25,-8.83,;18.25,-10.37,;16.92,-11.14,;16.92,-12.68,;15.59,-13.46,;14.26,-12.68,;12.92,-13.46,;12.92,-14.99,;11.58,-12.68,;11.58,-11.14,;12.92,-10.37,;10.25,-13.46,;8.92,-12.68,;8.92,-11.14,;7.58,-13.46,;7.58,-14.99,;8.92,-15.77,;10.25,-15,;11.57,-15.76,;11.57,-17.31,;12.9,-18.08,;10.24,-18.08,;8.92,-17.3,;6.25,-12.68,;4.91,-13.46,;4.91,-14.99,;3.58,-12.68,;3.58,-11.14,;2.25,-10.37,;2.25,-13.46,;.91,-12.69,;-.41,-13.46,;-1.75,-12.7,;-3.08,-13.47,;-4.4,-12.71,;-5.73,-13.48,;-7.06,-12.72,;-8.6,-12.71,;-10.14,-12.7,;15.59,-14.99,;14.26,-15.77,;16.92,-15.77,;16.92,-17.3,;15.59,-18.08,;15.59,-19.61,;14.26,-20.39,;12.92,-19.61,;14.26,-21.93,;18.25,-18.08,;19.59,-17.3,;18.25,-19.61,;19.59,-20.39,;19.59,-21.93,;18.25,-22.7,;18.1,-24.23,;16.59,-24.55,;15.82,-23.22,;16.86,-22.07,;20.93,-19.61,;20.93,-18.08,;22.26,-20.39,;23.59,-19.61,;23.59,-18.08,;24.93,-17.3,;26.26,-18.08,;27.59,-17.3,;27.59,-15.77,;26.26,-14.99,;24.93,-15.77,;24.93,-20.39,;24.93,-21.93,;26.27,-19.61,;27.6,-20.39,;27.6,-21.93,;28.93,-22.7,;28.93,-24.24,;30.27,-25.01,;30.27,-26.55,;28.93,-27.33,;31.6,-27.33,;28.93,-19.61,;28.93,-18.08,;30.27,-20.39,;31.6,-19.61,;31.6,-18.08,;32.94,-17.3,;34.34,-17.93,;35.38,-16.79,;34.61,-15.45,;35.09,-13.99,;34.06,-12.84,;32.55,-13.16,;32.07,-14.62,;33.1,-15.77,;32.94,-20.39,;32.94,-21.93,;34.28,-19.61,;35.61,-20.39,;36.94,-19.61,;36.94,-18.08,;38.28,-20.39,;39.61,-19.61,;39.61,-18.08,;38.28,-17.3,;38.28,-15.77,;39.61,-14.99,;39.61,-13.46,;40.95,-20.39,;42.28,-19.61,;40.95,-21.93,;39.7,-22.84,;40.17,-24.3,;41.71,-24.3,;42.2,-22.84,;43.49,-22.01,;43.5,-20.47,;44.82,-22.79,;46.16,-22.03,;46.17,-20.49,;47.51,-19.73,;44.84,-19.71,;47.49,-22.81,;47.47,-24.35,;48.83,-22.05,)|